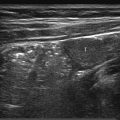
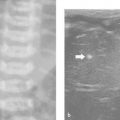
7 Pleura and Thorax
The use of ultrasonography of the thorax is limited by the presence of bone, air-containing structures (e.g., lungs, esophagus), and sometimes free air (e.g., subcutaneous emphysema, pneumothorax). Imaging of the chest with ultrasound is therefore confined to those structures that are not obscured by interposed aerated lung tissue or bony structures. Intrathoracic vascular structures such as the aorta, pulmonary arteries, and veins can be only superficially examined. For ultrasonography of the chest, a 15- to 17-MHz linear probe is used for the evaluation of superficial structures and pleural effusions. A 5- to 8-MHz curved probe can be used to analyze the mediastinum, intrathoracic structures, and pleural fluids. B-mode ultrasound is mostly used, in gray-scale and Doppler imaging. However, M-mode can be used to quantify motion of the diaphragm or to diagnose a pneumothorax.
The approach is either via a cervical, suprasternal/supraclavicular, intercostal/parasternal route or via a transdiaphragmatic view through the abdomen ( Fig. 7.1 , Fig. 7.2 , Fig. 7.3 ).
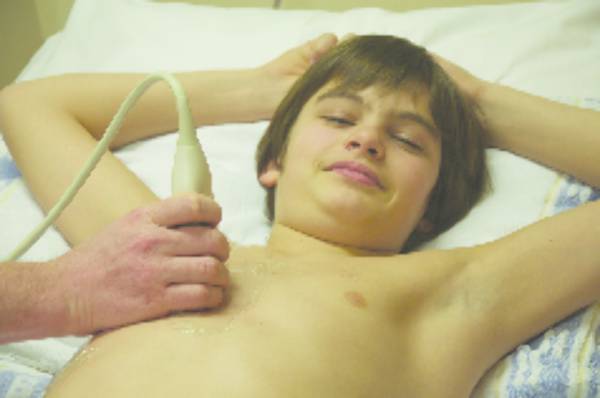
The advantage of using ultrasound in thorax imaging is its accessibility and bedside capabilities, which makes it a patient-friendly technique that can readily be used in emergency situations without the need for moving patients from the ward or emergency department. Furthermore, it allows direct intervention such as biopsy or pleural drainage ( Fig. 7.4 ; see also Chapter 18).
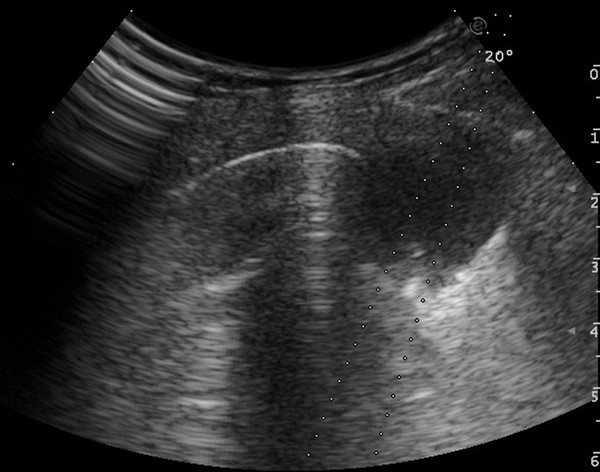
Since the general practice is that cardiac ultrasonography is performed by cardiologists rather than radiologists, imaging of the heart is not discussed in this chapter.
Ultrasound of the mediastinum is discussed in Chapter 6.
7.1 Indications for Ultrasonography
Ultrasonography of the chest can be indicated for an evaluation of pathology seen on a chest radiograph, in which case it allows a differentiation between pulmonary, pleural, and chest wall disease. A more common indication is to assess a superficial lesion that is palpable or visible on physical examination. In these cases, the findings at physical examination can be correlated with the ultrasonographic findings. Ultrasound is an easily accessible imaging technique for making an initial assessment of the lesion in question.
Tips from the Pro
Sometimes, respiratory movement can make ultrasonography difficult. Challenging a small child to hold his or her breath as if swimming under water can be helpful. “I bet you can hold your breath longer than I can.”
7.2 Anatomy and Normal Variants
7.2.1 Thoracic Wall
Subcutaneous fat, muscle, and unossified costochondral and sternal cartilage are easily accessible for ultrasonography. Muscle appears hypoechoic, with hyperechoic muscle fibers and tendons. Fat is hypoechoic, with connective tissue septa visible as hyperechoic strands. Unossified cartilage is hypoechoic, with variation in shape and size. Ossified bone is only superficially accessible, as only the cortex can be assessed.
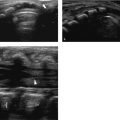
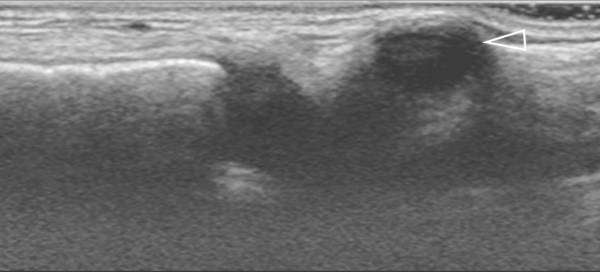
Ultrasound can also be used to discriminate between pathology and normal variants, as in patients with asymmetric development of the chest wall and cartilage ( Fig. 7.5 and Fig. 7.6 ) or a prominent xiphoid ( Fig. 7.7 ).
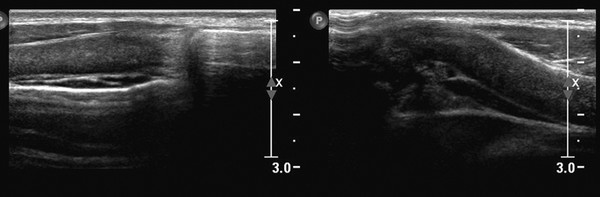
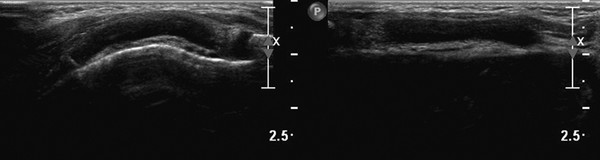
Tips from the Pro
Changes in shape of the chest wall can be hard to visualize. The use of dual-screen imaging in which both sides can be visualized side by side can be helpful. Panoramic views can be helpful, as well.
7.2.2 Pleura
The pleura itself under normal conditions is not visible on ultrasonograms. Ultrasound can, however, depict pleural thickening, pleural fluid, and a pneumothorax. Under normal circumstances, the pleural cavity may contain a small amount of fluid. Whereas chest radiographs will miss small amounts of fluid of up to 200 mL, ultrasound can easily detect them. Small amounts of fluid can best be visualized with the patient in a sitting position with the back toward the sonographer.
7.2.3 Lungs
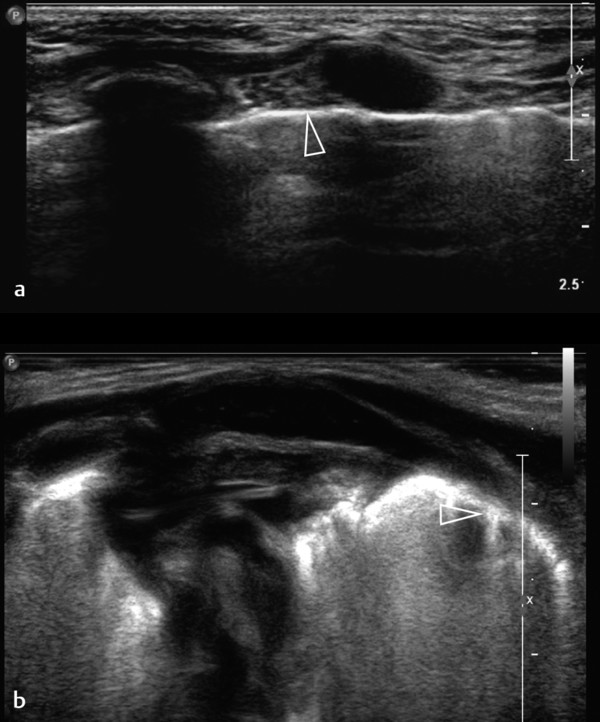
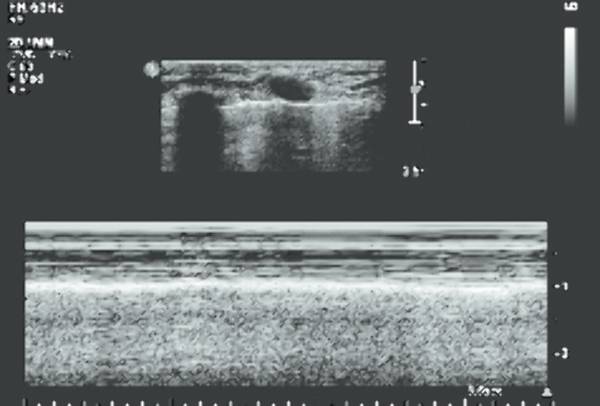
Normal, air-containing lung parenchyma is not accessible to ultrasound. The presence of air will cause a hyperechoic reflective surface with reverberation and comet tail artifacts ( Fig. 7.8 a,b; Video 7.8). One can appreciate the movement of lung and visceral pleura along the parietal pleura on inspiration, the “gliding sign.” On M-mode, this will produce a typical pattern known as “seashore sign” ( Fig. 7.9 ), which if present excludes a pneumothorax. In case of pulmonary consolidation or atelectasis, the lung parenchyma can be visualized.
7.2.4 Breast
For imaging of the breast in children, ultrasound is the method of choice. With high-frequency probes, excellent detailed imaging can be obtained. Mammography does not play a significant role in the imaging of pediatric breast lesions because of the denseness of the mammary glands and the use of radiation.
After birth, breasts can be enlarged in both boys and girls up to the age of 12 months under the influence of maternal hormones. On ultrasound, flaring of glandular tissue is typical ( Fig. 7.10 ). In prepubertal children, the breast is a collection of simple, branched, epithelium-lined ducts surrounded by connective tissue.
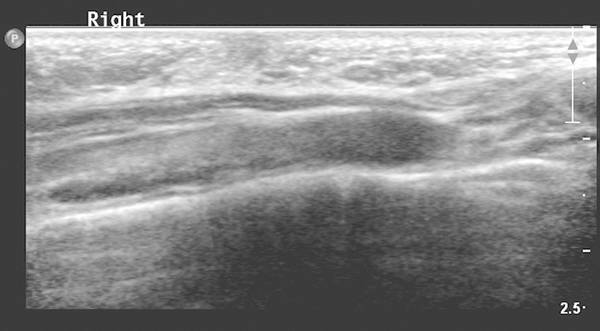
Physiologic breast development in girls occurs between 9 and 13 years of age under hormonal stimulation. Development can be divided in five Tanner stages ranging from subtle hyperechoic retroareolar tissue to fully developed breast tissue with echogenic fibroglandular tissue, hypoechoic ducts, Cooper ligaments, and hypoechoic fat (see Fig. 7.11 and Fig. 7.12 ). Changes in hormonal levels can lead to asymmetric breast development. Ultrasound is often used to exclude pathology ( Fig. 7.13 ).
7.2.5 Diaphragm
The diaphragm is visible as a thick echogenic line. This is best appreciated via the subxiphoid view in the parasagittal or coronal plane ( Fig. 7.14 ). Ultrasound can be used for both anatomical and functional analysis. Contour abnormalities (e.g., eventration and hernias) are visible on ultrasound. The most common anatomical variant is the diaphragmatic slip. This is characterized by a slip or fold of muscle of the diaphragm, which can cause a smooth indentation of the liver or spleen. Also, nodular crura and hypertrophy of the medial and lateral arcuate ligaments can be seen.
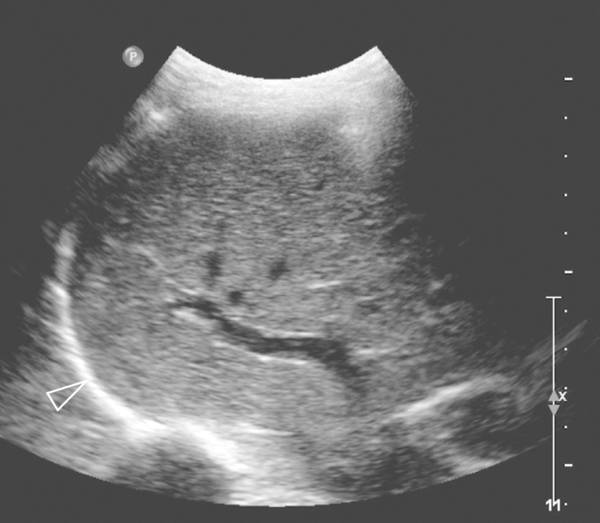
The movement of the diaphragm can be analyzed with M-mode ( Fig. 7.15 ; Video 7.15). It can visualize the direction of motion and the amplitude excursion of the diaphragm. For this technique two acoustic windows are used, oblique transverse subxiphoidal and intercostal. On inspiration the diaphragm moves toward the transducer, and on expiration movement is cephalad, away from the probe. This should be correlated with the phase of the respiratory cycle to determine normal or paradoxical movement. The amplitude is measured in the vertical axis. Movement should be reported as either normal, absent, or paradoxical. Normal movement of the diaphragm is over 4 mm on M-mode, and there should be less than a 50% difference in excursion between the two hemidiaphragms.
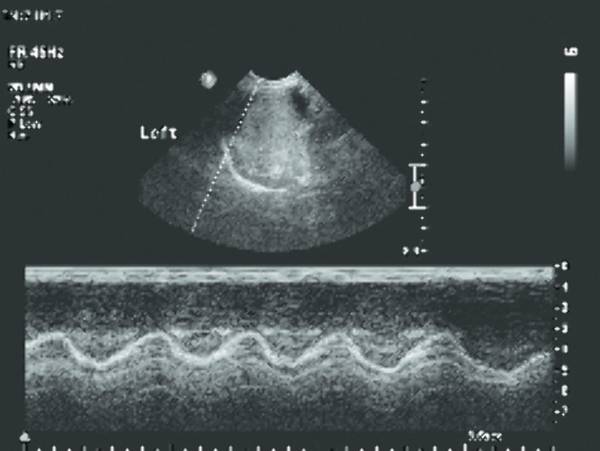
Tips from the Pro
With the use of M-mode, a reproducible quantification can be easily shown. Also, for the clinician, this is readily interpretable information.
7.3 Pathology
7.3.1 Chest Wall
Poland Syndrome
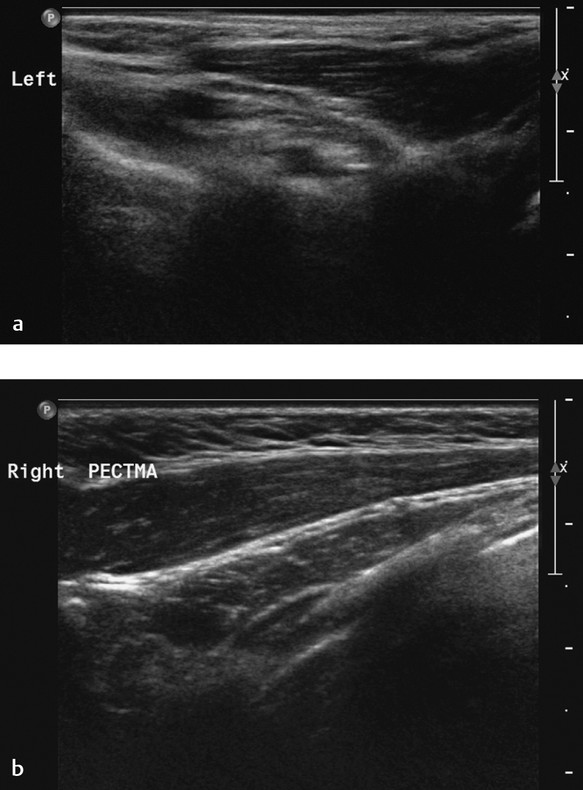
Poland syndrome (OMIM %173800) is hypoplasia of one side of the thorax. Features include hypoplasia of the breast, nipples, and subcutaneous tissue; lack of the pectoralis major muscle and minor musculature; and aplasia or deformity of the costal cartilages of ribs 2 through 4 or ribs 3 through 5. In addition, part of the syndrome can be alopecia of the axillary and mammary region in combination with ipsilateral brachysyndactyly. A possible cause of this syndrome may be developmental abnormality due to hypoplasia of the subclavian artery. The genetics of this disorder are not yet understood. The clinical manifestations can range from aplasia of the pectoral muscles alone to the complete spectrum of features. On ultrasound, an asymmetry of the chest due to hypoplasia or aplasia of the musculature should raise the possible diagnosis of of Poland syndrome ( Fig. 7.16 ).
Rib Anomalies
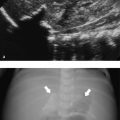
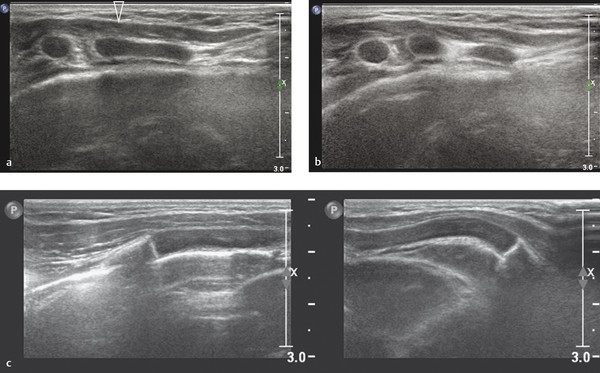
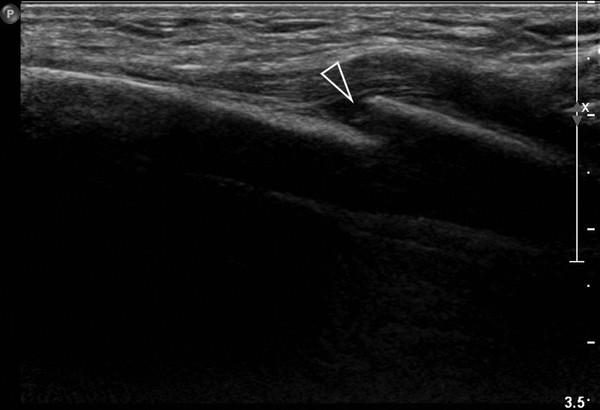
Asymptomatic swelling and asymmetry of the chest will often be due to an abnormal development of ribs, especially of the costal cartilage. In these patients, chest radiographs will often be requested by the referring physicians. However, since the asymmetry is most often located in the cartilaginous part of the rib cage, thoracic ultrasound should be the imaging method of first choice. With the help of ultrasound, it is possible to exclude underlying pathology in most patients. Thoracic ultrasound can show developmental abnormalities of the cartilage and ribs such as forked ribs ( Fig. 7.17 ; Video 7.17a,b), fusion of ribs ( Fig. 7.18 ; Video 7.18), and cervical ribs.
Trauma
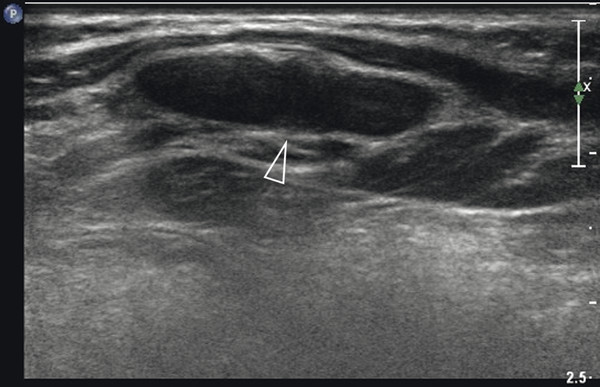
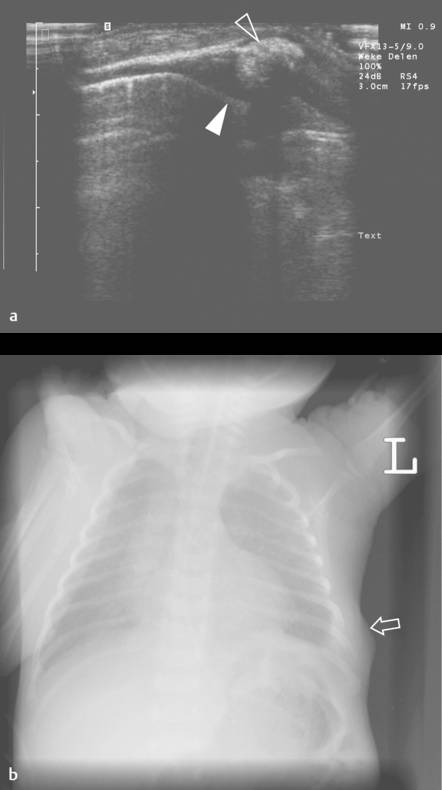
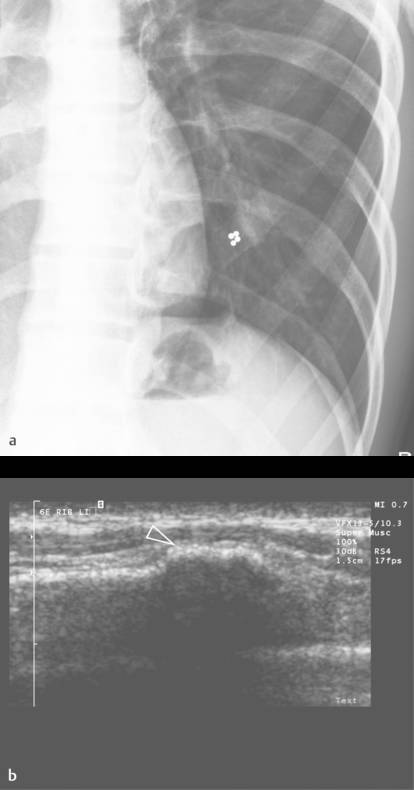
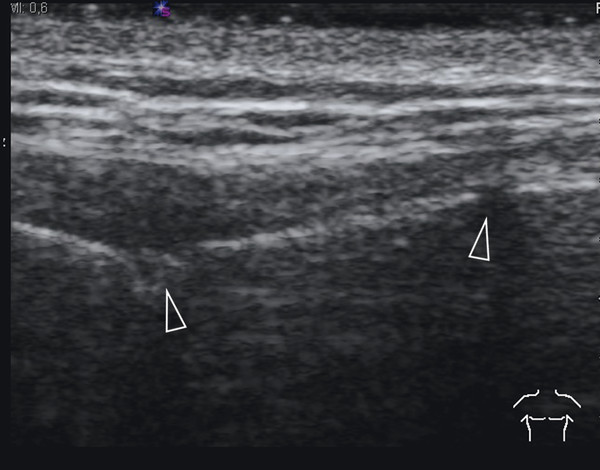
Although not the imaging method of choice, ultrasound can be used to visualize fractures of the ribs and sternum. In the acute phase, a fracture will produce a cortical disruption, sometimes surrounded by a hematoma ( Fig. 7.19 ). In a later phase, callus formation can be seen ( Fig. 7.20 and Fig. 7.21 ). Hematoma can cause a broad spectrum of nonspecific sonographic features ( Fig. 7.22 ). Moreover, hematomas are usually clinically evident. Therefore, they are not discussed here.
Infectious Lesions
Chest wall infection in children is uncommon. Spread is either hematogenous or via direct infection from surrounding tissue (e.g., after chest surgery). Infectious agents include Staphylococcus aureus, Mycobacterium tuberculosis, Actinomyces, Nocardia, Aspergillus, and Candida. Fungal infections usually occur in immunocompromised patients (e.g., during chemotherapy).
Infectious processes of the chest wall, like other soft-tissue infections, can appear as a complex mass with or without abscess formation. A heterogeneous mass with hypervascularity can be seen on Doppler ultrasound with infiltration of the surrounding fat. Also, destruction of ribs can be seen on ultrasound.
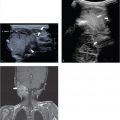
In case of a chest wall abscess, ultrasound will show an encapsulated, hypoechoic, sometimes heterogeneous mass. The mass can have posterior acoustic enhancement. Doppler can show peripheral flow surrounding the lesion. Abscess formation can also occur around surgically implanted materials such as a Nuss bar ( Fig. 7.23 and Fig. 7.24 ). Magnetic resonance (MR) imaging or computed tomography (CT) is helpful when there is suspicion of expansion into more deeply positioned structures or intrathoracic involvement. In cases of osteomyelitis, there is no role for ultrasound. Only in complications such as abscess formation ultrasound can play a role .
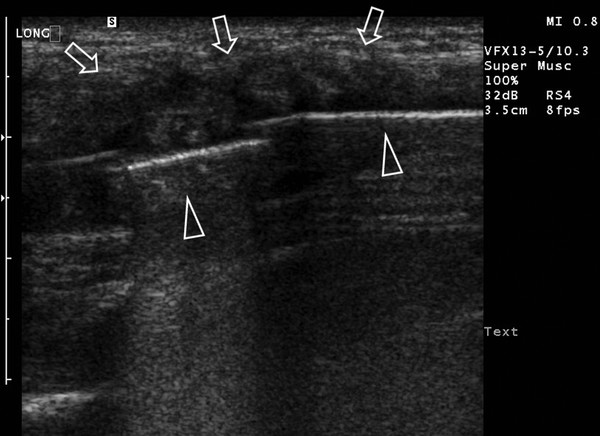
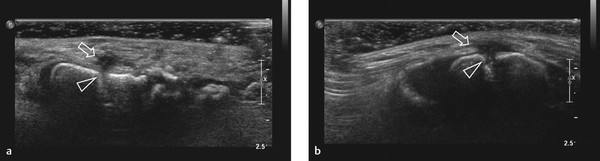
Self-Limiting Sternal Tumor of Childhood
In young children presenting with a fast-growing tumor over the sternum, a self-limiting sternal tumor of childhood (SELSTOC) should be considered in the differential diagnosis. Children can present with or without signs of infection. In the latter case, the main concern will be the presence of a tumor. In the majority of children, the presenting symptom is pain, and discoloration is visible in 50% of reported patients. Generally, laboratory examinations are normal, and microbiologic cultures are negative in all reported cases. Ultrasound shows a well defined, dumbbell-shaped, hypoechoic, heterogeneous, poorly vascularized lesion ( Fig. 7.25 ). The lesion has no contact with the skin and does not infiltrate bone or muscle. As a result of the dumbbell shape and involvement of the sternal cartilage, the distance between the sternal ossification centers can be increased. When ultrasound imaging is typical of SELSTOC, a wait-and-see policy can be implemented. Follow-up via ultrasonography is not necessary but can be reassuring for the parents, who can be afraid of malignancy, and treating physicians, who may not be aware of this entity.
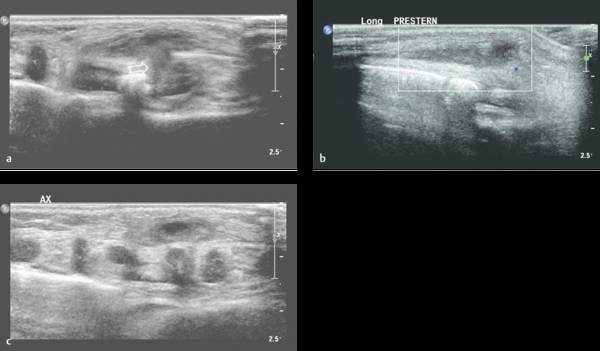
Lymphadenopathy
Lymphadenopathy can be seen in reaction to all kinds of (superficial) infections (e.g., in cat-scratch disease). The differentiation between reactive lymphadenopathy and malignant lymph nodes is based mainly on the presence of a fat center and an oval shape in reactive lymphadenopathy ( Fig. 7.26 , Fig. 7.27 , Fig. 7.28 ).
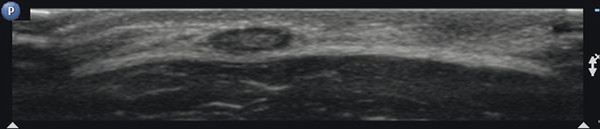
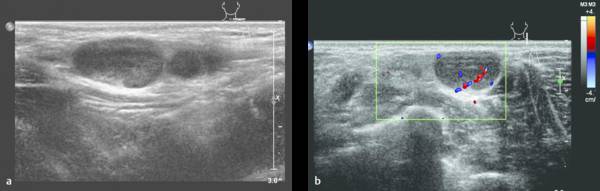
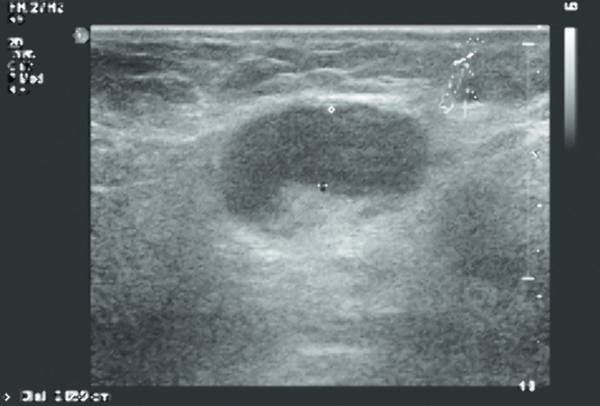
In the course of infectious disease, abscess formation in lymph nodes is possible. This will present as lymph nodes with central liquefaction and surrounding hyperemia.
Tips from the Pro
If there is concern about fluctuation during ultrasonography, try to keep the ultrasound probe stable while gently compressing the collection with your free hand.
Tumors of the Chest Wall
Tumors of the chest wall are rare, and in contrast to those in adults, chest wall tumors in children are mainly primary tumors. Most chest wall tumors are malignant. In most cases, ultrasound will be the initial imaging technique. Often, the imaging characteristics will be nonspecific, but a combination of the patient’s history and age, location of the tumor, and imaging characteristics can be helpful for making a differential diagnosis. A practical diagnostic approach is to categorize the tumors based on their origin as either soft-tissue tumors or bone tumors.
Soft-Tissue Tumors of the Chest Wall
Rhabdomyosarcoma
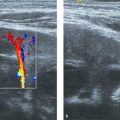
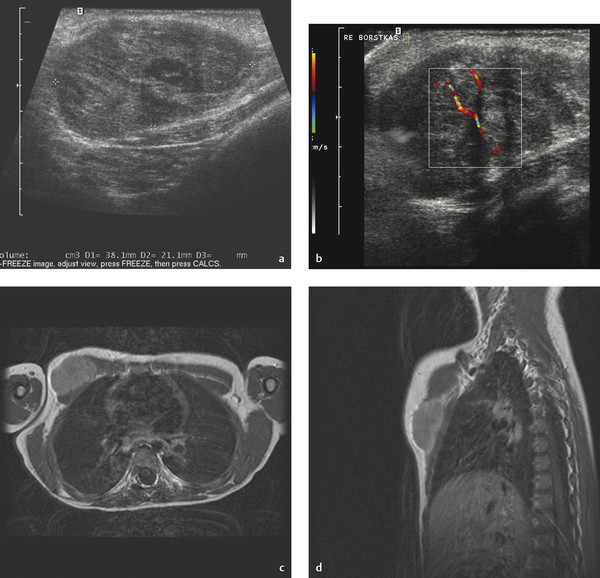
Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and, after Ewing sarcoma, the most common chest wall malignancy in childhood. The prognosis and survival of a patient with rhabdomyosarcoma depend on the site of origin and histology. Chest wall rhabdomyosarcoma tends to have a poor prognosis. The initial presentation is often a soft-tissue swelling, and the clinical symptoms depend mainly on the structures involved. Ultrasound will show a lesion without specific characteristics, usually heterogeneous and poorly defined with flow in the periphery. Biopsy is necessary for diagnosis. In all cases, additional MR imaging will be mandatory to assess the extent of the tumor and its relation to surrounding anatomical structures such as nerves and vessels ( Fig. 7.29 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree