8 Peritoneal Cavity and Retroperitoneal Space
In this chapter, the peritoneal cavity and the retroperitoneal space will be discussed. The focus is solely on the anatomical region and not on the organs within (as these are discussed in separate chapters in this book).
8.1 Normal Anatomy
The peritoneal cavity is the space between the parietal peritoneum and the visceral peritoneum, which covers all the intraperitoneal organs (i.e., the stomach, spleen, gallbladder, liver, and part of the intestines). Approximately 50 mL of fluid is produced daily and circulates throughout the peritoneal cavity in a well defined pattern. During the evaluation of patients with a possible malignancy, it is important to be aware of this pattern of circulation because there are certain areas of stasis where small deposits of, for example, malignancies can be found ( Fig. 8.1 ). Resorption of the peritoneal fluid is predominantly achieved in the subphrenic space.

It is important to be aware of the fact that whereas in boys the peritoneal cavity is a completely enclosed environment, in girls there is an opening at the level of the ovarian tubes. This can serve as a port of entry for infections such as pelvic inflammatory disease.
The retroperitoneal space is the anatomical region behind the peritoneum, and it contains several organs, which can be remembered with the mnemonic SAD PUCKER ( Table 8.1 ). The retroperitoneal area surrounding the kidneys is divided into three separate parts. The perirenal space is bounded by the Zuckerkandl fascia on the posterior side and the Gerota fascia on the anterior side. This space contains the kidney, adrenal gland, and renal vessels. The anterior pararenal space is bounded posteriorly by the Gerota fascia and anteriorly by the posterior peritoneum. It contains the retroperitoneal duodenum, pancreas, and ascending and descending colon. Finally, the posterior pararenal space, which contains only fat, is bounded by the muscles of the posterior abdominal wall posteriorly and the Zuckerkandl fascia anteriorly.
S | Suprarenal glands |
A | Aorta/inferior vena cava |
D | Duodenum (second and third segments only) |
P | Pancreas (tail is intraperitoneal) |
U | Ureters |
C | Colon (ascending and descending colon only) |
K | Kidneys |
E | Esophagus |
R | Rectum |
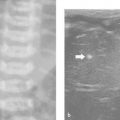
In children, the retroperitoneum can easily be visualized with ultrasonography. In most cases, a graded compression technique and a high-frequency linear transducer can be used ( Fig. 8.2 ).

8.2 Pathology
8.2.1 Abdominal Vessels
Aorta
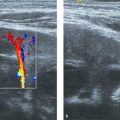
In contrast to adults, pathology of the abdominal aorta in children is rare. There are only a handful of reported cases of congenital anomalies of the abdominal aorta, consisting mainly of atresia and duplication. Other reported pathologies of the abdominal aorta include middle aortic syndrome ( Fig. 8.3 and Fig. 8.4 ), Takayasu arteritis, thrombosis ( Fig. 8.5 ), and aneurysms. Of special interest are the long-term survivors of abdominal neuroblastoma. There have been reports of midaortic syndrome in these children, both in those who have and in those who have not had radiation therapy.



In all of these rare cases, additional imaging with computed tomography (CT) and/or magnetic resonance (MR) imaging is mandatory.
Inferior Vena Cava
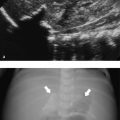
Congenital anomalies of the inferior vena cava (IVC) are in general an incidental finding and do not warrant specific follow-up. Because of the complex embryology of the IVC (which comprises four different segments: hepatic, suprarenal, renal, and infrarenal), multiple variants can be encountered. The most common variant is absence of the hepatic segment of the IVC, leading to the azygos continuation ( Fig. 8.6 ; Video 8.6). Other variants include left-sided IVC, double IVC, and anomalous/multiple origins of the veins draining into the IVC ( Fig. 8.7 and Fig. 8.8 ).



Knowledge of the normal variants of the IVC is important in differentiating between left-sided para-aortic masses and an aberrant IVC and, in cases of percutaneous venous intervention, in IVC stent placement.
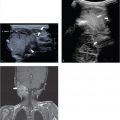
Thrombosis/occlusion of the IVC can be encountered in children with central lines and in oncology patients (especially children with Wilms tumor; Fig. 8.9 ; Video 8.9 and Fig. 8.10 ). Ultrasound (US) is in these cases the imaging method of choice. The optimal way to visualize the IVC is from the front. However, in some children this will yield a suboptimal view because of such factors as body shape and gas-distended bowels. In such cases, a lateral approach to the IVC can be used, in which the retroperitoneum is used as a window.


8.2.2 Lymphadenopathy
Mesenteric Lymphadenopathy
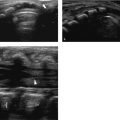
The mesenteric lymph nodes in children are easily visualized, and to the inexperienced radiologist they can look like a pathologic finding. The normal short-axis diameter of the mesenteric lymph nodes is up to 8 mm ( Table 8.2 ). Mesenteric lymphadenopathy is commonly seen in mesenteric adenitis, which is most often caused by a viral infection. However, enlarged, ovoid lymph nodes can also be seen in a significant proportion of otherwise healthy children ( Fig. 8.11 and Fig. 8.12 ). The sole finding of such lymph nodes should not lead to a further work-up. If, however, the lymph nodes show a change in texture, with loss of the hilum and a round, hypoechoic appearance, further analysis is mandatory, either by additional work-up, including cross-sectional imaging, or by biopsy ( Fig. 8.13 ). In patients with large and bulky lymph nodes, further work-up is mandatory. In many cases, a percutaneous or a surgical biopsy must be performed.
Threshold size (mm) | No. of enlarged lymph nodes | False-positive rate (%) |
≥ 5 | 33 | 54 |
> 6 | 18 | 30 |
> 7 | 8 | 13 |
> 8 | 3 | 5 |
> 9 | 3 | 5 |
> 10 | 0 | 0 |
Source: Reprinted with permission from Karmazyn B, Werner EA, Rejaie B, Applegate KE. Mesenteric lymph nodes in children: what is normal? Pediatr Radiol 2005;35(8):774–777. Note: In this retrospective study, 61 children (36 boys and 25 girls; mean age, 10.7 years; range, 1.1–17.3 years) underwent noncontrast abdominal computed tomography for the evaluation of suspected or known renal stones; abdominal lymph node size was evaluated. Enlarged mesenteric lymph nodes (short axis > 5 mm) were found in 33 (54%) of the 61 children. The majority of the enlarged mesenteric lymph nodes was found in the right lower quadrant (88%). Based on their findings, the authors stated that a short-axis diameter of > 8 mm might be a more appropriate definition for mesenteric lymphadenopathy in children. | ||



Retroperitoneal Lymphadenopathy
Unlike mesenteric lymphadenopathy, enlargement of the retroperitoneal lymph nodes should always be regarded as abnormal ( Fig. 8.14 ). Therefore, this finding warrants further follow-up. In children, the most common causes of retroperitoneal lymphadenopathy are malignancies, such as Wilms tumor, neuroblastoma, rhabdomyosarcoma, and malignant lymphoma; however, infectious diseases can also cause lymph node enlargement.

On US, lymphadenopathy can be obscured by gastrointestinal gas or, in an increasingly large group of children, obesity. In children with large abdominal tumors, the retroperitoneum can be difficult to visualize. In these children, disease staging with additional imaging (e.g., MR imaging) is mandatory.
Tips from the Pro
If the retroperitoneum is obscured by intestinal gas, graded compression, as in the diagnosis of appendicitis, should be applied. Compression should be applied during inspiration.
8.2.3 Intraperitoneal Fluid Collections
Ascites
Ascites is the accumulation of free fluid in the peritoneal cavity. In healthy girls, a small amount of fluid in the Douglas pouch is a normal physiologic finding ( Fig. 8.15 ). In all other cases, ascites should be regarded as a pathologic finding.

The causes of ascites can be differentiated according to whether a transudate, with a low protein content, or an exudate is present. The most common cause of a transudate is portal hypertension, and therefore a transudate is seen in children with cirrhosis and heart failure ( Fig. 8.16 ; Video 8.16). A transudate can also be seen in severe malnutrition (kwashiorkor) and nephrotic syndrome. An exudate is seen primarily in peritoneal metastatic disease and infection ( Fig. 8.17 , Fig. 8.18 , Fig. 8.19 , Fig. 8.20 ; Videos 8.19 and 8.20). There is, however, a large differential diagnosis for the presence of ascites, which is different for neonates ( Table 8.3 ) and children ( Table 8.4 ; Fig. 8.21 and Fig. 8.22 ).
Hepatobiliary disorders | Genitourinary disorders |
Cirrhosis | Obstructive uropathy |
Alpha1-antitrypsin deficiency | Posterior urethral valves |
Congenital hepatic fibrosis | Ureterocele |
Viral hepatitis | Lower ureteral stenosis |
Budd–Chiari syndrome | Ureteral atresia |
Biliary atresia | Imperforate hymen |
Bile duct perforation | Bladder rupture |
Portal venous malformation | Bladder injury from umbilical artery catheterization |
Ruptured mesenchymal hamartoma | Nephrotic syndrome |
Gastrointestinal disorders | Ruptured corpus luteum cyst |
Intestinal malrotation | Cardiac disorders |
Intestinal perforation | Arrhythmia |
Acute appendicitis | Heart failure |
Intestinal atresia | Hematologic disorder |
Pancreatitis | Neonatal hemochromatosis |
Chylous ascites | |
Parenteral nutrition | |
Extravasation | |
Metabolic disease | Other |
Congenital cutis marmorata telangiectatica | |
Intravenous vitamin E | |
Pseudoascites | |
Small-bowel duplication | |
Abdominal trauma | |
Idiopathic | |
Source: Reprinted with permission from Giefer MJ, Murray KF, Colletti RB. Pathophysiology, diagnosis, and management of pediatric ascites. JPGN 2011;52(5):503–513. | |
Hepatobiliary disorders | Neoplasm |
Cirrhosis | Lymphoma |
Congenital hepatic fibrosis | Wilms tumor |
Acute hepatitis | Clear cell renal sarcoma |
Budd–Chiari syndrome | Glioma |
Bile duct perforation | Germ cell tumor |
Liver transplantation | Ovarian tumor |
Mesothelioma | |
Neuroblastoma | |
Gastrointestinal disorders | Metabolic disease |
Acute appendicitis | Genitourinary disorders |
Intestinal atresia | Nephrotic syndrome |
Pancreatitis | Peritoneal dialysis |
Pyloric stenosis | Cardiac disorder |
Serositis | Heart failure |
Crohn disease | Pseudoascites |
Eosinophilic enteropathy | Celiac disease |
Henoch-Schönlein purpura | Cystic mesothelioma |
Chylous ascites | Omental cyst |
Intestinal lymphangiectasia | Ovarian cyst |
Lymphatic duct obstruction | Other |
Lymphatic duct trauma | Systemic lupus erythematosus |
Parenteral nutrition extravasation | Ventriculoperitoneal shunt |
Vitamin A toxicity | |
Chronic granulomatous disease | |
Nonaccidental trauma | |
Idiopathic | |
Source: Reprinted with permission from Giefer MJ, Murray KF, Colletti RB. Pathophysiology, diagnosis, and management of pediatric ascites. JPGN 2011;52(5):503–513. | |







Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree