N
SLN detection rate
Blue dye (%)
ICG (%)
Tagaya [7]
25
92
100
Abe [8]
128
65.4
100
Sugie [9]
99
78
100
Hojo [10]
113
93
100
Van der Vorst [11]
24
84
100
Shasfsma [12]
32
88
100
Wichart [13]
100
99
100
10.2.2 Comparison Between ICG Fluorescence and RI
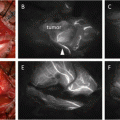
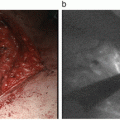
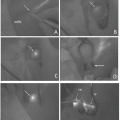
Dual mapping involved with RI and blue dye is currently the standard technique for SLN localization. The detection rate for this technique was 96 % with low false-negative rate of 7.3 % [15]. Regardless of this clinical usefulness, the RI method has some drawbacks as mentioned above and cannot be uptaken in the small-volume center without involvement of nuclear medicine. As shown in Table 10.2, previous studies reported that ICG fluorescence was comparable or superior to RI for overall SLN detection [10–13, 16–19]. Two studies reported that SLN detection rate for ICG was 100 % compared with 88-89 % for RI and 78-88 % for blue dye [11, 12]. Wishart GC and colleagues [13] used tree tracer agents (ICG, RI, and blue dye) and compared SLN detection between these three techniques. In their study, the detection rates were 91.3 % for RI, 99 % for blue dye, and 100 % for ICG, and the combination of ICG and blue dye had the highest nodal sensitivity at 95.0 %. As blue dye does not have any impact for SLN detection when ICG is used simultaneously, the ICG fluorescence method has a potential to be an alternative to a dual technique with RI and blue dye. On the basis of these results, ICG fluorescence method alone is applicable as clinical practice if access to RI is not available.
Table 10.2
Surgical outcome of SLN biopsy using RI and ICG
N | SLN detection rate | ||
|---|---|---|---|
RI (%) | ICG (%) | ||
Hojo [10] | 113 | 100 | 93.1 |
Van der Vorst [11] | 24 | 95.8 | 95.8 |
Shasfsma [12] | 32 | 100 | 100 |
Wishart [13] | 100 | 91.3 | 100 |
Murawa [16] | 20 | 85 | 100 |
Ballardinni [17] | 134 | 99.2 | 100 |
Verbeek [18] | 95 | 97.9 | 98.9 |
Samorani [19] | 301 | 95.3 | 98.6 |
10.2.3 Clinical Issue for the ICG Fluorescence Method
One drawback of the ICG fluorescence method is attenuation of fluorescence signal. As emitted fluorescence from ICG is scattered through fat droplets, detection of fluorescence signal deeper than 1 cm is difficult. As RI is superior to ICG fluorescence for tissue penetration, patients with high BMI is better subject for the RI method. Some previous studies reported that BMI was correlated with lower detection rate and longer time to identify SLN [8, 11]. In other studies, however, the detection rate for ICG fluorescence is independent of BMI and yields high detection rate regardless of high BMI [9, 19].
The second disadvantage was the larger number of SLN removed for the ICG fluorescence method. In previous studies, the mean number for SLN removed using ICG fluorescence ranged from 1.5 to 5.4 which was significantly higher than that using RI and/ or blue dye. ICG’s small molecular size and spilt of ICG in the operation field might cause the larger number of fluorescence positive lymph nodes. To obtain an optimal concentration of ICG, Mieog JS and colleagues [20] examined the signal to background ratio (SBR) at the various concentration of human serum albumin-conjugated ICG (ICG: HSA), and they obtained the highest SBR at the concentration of 400–800 μM. The other study for melanoma also reported that a dose of 600 μM ICG: HAS was optimal to obtain high SBR [21]. A dose of 0.5 % (6.4 mM) which was widely used is approximately 10-fold higher than the optimal dose reported. Because the standard dose and concentration of ICG have not been determined yet, a larger study to address the optimal technique may be required.
10.3 Future Direction
SLN biopsy after neoadjuvant chemotherapy (NAC) is still controversial in terms of accuracy for axillary staging. The previous multicenter trial reported that the detection rate for SLN after NAC was 88.9 % for RI alone, 78.1 % for blue dye, and 87.6 % for the combination of RI and blue dye [22]. Three meta-analyses addressing accuracy for SLN biopsy after NAC reported that the RI and/or blue dye method provided a detection rate of 89.6–90.9 % and a false-negative rate of 8.4–12 % [23–25]. Updated ASCO clinical practice guideline reported that SLN may be offered after NAC [26]. The RI method after NAC, however, had a trend for a lower detection rate than before NAC. In the SENTIA trial [27], a false-negative rate for SLN mapping was 14.2 % for the patients who converted from clinically node-node positive to node-negative disease after NAC. This impairment of the SLN detection might be related with fibrosis and lymphatic obstruction by cytotoxic reagents.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree