The abdominal aorta and its primary branches are involved in many diseases and conditions that affect large and medium-sized arteries. Traditional evaluation of the abdominal aorta relied on catheter aortography; however, with the advent of multislice computed tomography (CT), rapid and robust CT angiography (CTA) imaging of the abdominal aorta and its branches has increasingly rivaled catheter angiography as a primary diagnostic imaging modality, particularly in hemodynamically stable patients. Knowledge of the radiologic appearance of the abdominal aorta and branches is helpful in differentiating various pathologic states.
Key Issues for Problem Solving in Imaging of the Abdominal Aorta and its Branches
- ▪
Features of occlusive disease
- ▪
Differentiating aneurysmal disease
- ▪
Imaging the postoperative aorta
- ▪
Differentiating causes of the acute aortic syndrome
- ▪
Features of iatrogenic and traumatic disease
- ▪
Findings in inflammatory vascular disease
- ▪
Imaging the visceral arteries
Occlusive Disease
Aortoiliac Atherosclerosis
Mural calcification of the abdominal aorta and primary branches is a common finding on CT scanning. In fact, the prevalence of calcific changes in the abdominal aorta has been reported to be more than 90% in certain ethnic groups by age, including non-Hispanic whites and Chinese populations. Common causes of mural calcification include hypercholesterolemia, diabetes, smoking history, hypertension, and renal disease. Atherosclerotic aortoiliac stenotic or occlusive disease has been reported to occur in slightly more than half of all patients who are evaluated for peripheral arterial disease.
Although identifying changes of atherosclerosis is typically not a diagnostic imaging dilemma, certain features are helpful in assessing the significance of the affected arterial segments. For example, chronic aortoiliac occlusive disease is characterized by numerous collateral pathways for reconstitution of the pelvic vessels and the lower extremity runoff. Dominant collateral pathways are typically through arteries running along the body wall, such as the internal mammary arteries, epigastric arteries, and lumbar arteries, or by arteries supplying viscera, such as branches of the inferior mesenteric artery. These structures are best seen by angiography, but the astute imager also recognizes many of these pathways on CT or magnetic resonance imaging (MRI) as abnormally dilated collateral vessels ( Fig. 46-1 ). Without the real-time benefit of flow dynamics seen on catheter angiography, the presence of these collateral vessels is a good cross-sectional clue to severe focal stenosis or occlusion. Focal stenosis or occlusion can be missed in the axial dimension if the lesion is very short and good collateral runoff opacifies the vessel distal to the diseased segment. Evaluation of reformatted images in the coronal and sagittal planes should therefore be performed because a focal hemodynamically significant lesion, which was otherwise subtle on axial CTA, may be better demonstrated. Common sites of such lesions in the peripheral circulation include the superficial femoral artery at the adductor hiatus, where short focal lesions are often associated with dilated collateral vessels through the profunda femoris or genicular branches to supply the runoff. Viewing well-opacified distal runoff vessels in this setting as indicative of no significant disease would be a mistake.

Coral Reef Aorta
One particularly significant form of aortic occlusive disease is known as the “coral reef” aorta, so named because of the bulky, frondlike calcifications seen primarily to involve the visceral segment of the abdominal aorta ( Fig. 46-2 ). This condition may result in hemodynamically significant stenoses of the visceral and mesenteric arteries, as well as compromised flow to the lower extremities. Recognizing this finding is important because it may be a cause of significant distal embolic disease. In addition, given that the coral reef aorta increases the risk of embolic disease during endovascular procedures, awareness of this finding is important for the interventionalist.

Rare Entities
Nonatherosclerotic forms of aortic stenosis are rare. These include the coarctation or middle aortic syndromes, such as Takayasu arteritis, neurofibromatosis, mucopolysaccharidoses, and the hypoplastic aortic syndrome. Differentiating features of these conditions include the relative lack of atherosclerotic change, although concurrent atherosclerosis may still be present, and the younger age of the affected individuals. Some conditions, such as the hypoplastic aortic syndrome, have a predilection for young to middle-aged female patients ( Fig. 46-3 ). Other rare causes of nonatherosclerotic aortic stenosis include retroperitoneal fibrosis and radiation aortitis. Retroperitoneal fibrosis can be recognized by stranding and cicatrization in the surrounding retroperitoneal fat, classically associated with medial displacement of the ureters and possible hydronephrosis. Radiation aortitis can usually be suggested from the patient’s history. Aortic angiosarcoma can manifest with peripheral or visceral ischemia and a polypoid intraluminal mass on imaging. This mass may appear as polypoid thrombus and plaque and can be difficult to distinguish by CT. Clues to the diagnosis include an atypical polypoid appearance and lack of extensive atherosclerotic change or aneurysm. MRI with contrast is optimal for soft tissue characterization and enhancement.

Pearls and Pitfalls
- ▪
Aortoiliac stenotic and occlusive disease is common in patients with peripheral arterial disease.
- ▪
Atherosclerosis is the most common cause, but other rare entities should be entertained in certain demographic groups such as younger individuals.
- ▪
The coral reef aorta poses an increased risk of thromboembolic disease during intervention.
Aneurysmal Disease
Degenerative Abdominal Aortic Aneurysm
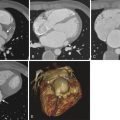
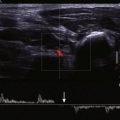
Degenerative aneurysmal disease of the abdominal aorta is thought to affect approximately 8% of the population, men much more commonly than women. The infrarenal abdominal aorta is primarily involved. The definition of aneurysm is generally accepted as a vessel that is more than 1.5 times its normal diameter. Therefore, for a typical 2-cm abdominal aorta, an aneurysm is defined as an aorta with a maximal cross-sectional diameter of more than 3 cm. Because the normal abdominal aorta tapers as it approaches the iliac bifurcation, an infrarenal abdominal aorta that does not meet size criteria for aneurysm but does not appropriately taper may herald early aneurysmal change. Although abdominal aortic aneurysm was originally thought to represent a sequela of atherosclerosis, it may represent a focal manifestation of a generalized vascular disease. Atherosclerotic changes are often concurrently present. CTA is the modality of choice for evaluation and follow-up of abdominal aortic aneurysms. CT clearly demonstrates aneurysm size and other potentially significant associated findings such as mural thrombus, calcifications, focal ulceration, or dissection ( Fig. 46-4 ). In addition, CT allows for evaluation of associated structures, such as the retroperitoneum for signs of hemorrhage or inflammation, the kidneys, and the ureters. Retroperitoneal stranding and high-density fluid in a patient presenting with acute back pain should suggest a ruptured or leaking abdominal aortic aneurysm ( Fig. 46-5 ). Contrast-enhanced CT may also demonstrate extravasation of contrast material or a focal outpouching from the aneurysm. Ultrasound is also useful as an imaging modality with its ability to demonstrate cross-sectional size and mural thrombus ( Fig. 46-6 ). Radiographs are less reliable for primary evaluation because they mainly rely on intimal calcification to delineate the aneurysm sac.



Multiplanar CT has become very helpful in preprocedural planning for endovascular aneurysm repair (EVAR), and several proprietary software products exist for accurately sizing graft components. A crucial aspect of evaluating aneurysm size is understanding that the aneurysmal process affects both aortic diameter and length. Tortuosity is therefore a frequent finding with aneurysmal dilation. As a result, care must be taken to measure the diameter of the aneurysm in its true orthogonal plane, rather than in the cross-sectional image seen on a representative CT slice. Obliquities in the plane of view can lead to gross overestimation and measurement variability. For this reason, using the current multiplanar features in electronic imaging is important for assessing true aneurysm diameter ( Fig. 46-7 ). Most interventionalists and surgeons believe that the risk of aneurysm rupture at 5 cm equals or exceeds the risk of procedural complications, and they therefore recommend treatment for aneurysms that reach that size threshold. For treatment planning, particularly for EVAR, certain measurements, such as diameter and length of the infrarenal neck, angulation of the neck, and diameter and length of the iliac arteries, are of paramount importance for assessing the compatibility of the aneurysm for endovascular repair and for sizing of the appropriate components ( Fig. 46-8 ). As technology and device engineering improve, these measurements will also change, and each manufacturer will have its own measurement profile for the device’s instructions for use. Because of this variability, even though reporting the relevant measurements is appropriate, the diagnostic radiologist should avoid commenting on suitability for endovascular repair in the radiology report.


Mycotic Abdominal Aortic Aneurysm
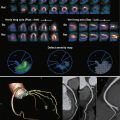
Other rare causes of abdominal aortic aneurysm formation include mycotic aneurysm, pseudoaneurysm, inflammatory aneurysm, and connective tissue disease related aneurysm. A mycotic aneurysm can arise either from infectious destruction of the aortic wall leading to mural weakening and aneurysmal degeneration or from secondary infection of a preexisting aneurysm. Mycotic aneurysms are typically irregular, lobulated, and saccular in morphology, and they can have perivascular inflammatory changes. These morphologic findings in a rapidly expanding aneurysm should raise the suspicion of an infectious origin. Common organisms include Salmonella and Staphylococcus species, and risk factors include vascular intervention, intravenous drug use, and immunosuppression including from diabetes or steroid use. If a mycotic aneurysm is suspected by clinical findings and cross-sectional morphology, a useful problem-solving modality would be an indium-111–tagged leukocyte scan to look for uptake in the aneurysm wall ( Figs. 46-9 and 46-10 ). This technique is also useful for identifying suspected surgical graft infection.


Inflammatory Abdominal Aortic Aneurysm
Inflammatory aortic aneurysms are associated with an elevated erythrocyte sedimentation rate, and they demonstrate a thickened mural rind surrounding the aneurysm. Occasionally, the rind enhances on contrast-enhanced CT and MRI during delayed phase imaging. This mural thickening can resolve after treatment ( Fig. 46-11 ). Recognizing and reporting features of an inflammatory aneurysm are important because these patients often have associated adhesions to the bowel and ureters that may make surgery more complicated. A very rare cause of inflammatory aneurysm caused by immunoglobulin G4–mediated disease has also been described ( Fig. 46-12 ).


Abdominal Aortic Pseudoaneurysm
Pseudoaneurysms of the abdominal aorta are typically sequelae of iatrogenic or blunt abdominal trauma. What differentiates a pseudoaneurysm from a true aneurysm is lack of all three components of the vascular wall. Pseudoaneurysms are typically contained only by adventitia, and as such they carry a significant risk of rupture. Pseudoaneurysm of the abdominal aorta may be a late manifestation of remote abdominal trauma. Iatrogenic causes include aortic surgery and spinal surgery. Finding an aneurysm adjacent to an anastomotic margin or area of spinal instrumentation therefore can be a clue to pseudoaneurysm ( Fig. 46-13 ).

Ultrasound, if performed, may show typical to and fro flow on Doppler imaging characteristic of pseudoaneurysms elsewhere in the body. This finding is likely less specific for an abdominal aortic pseudoaneurysm, however, because any saccular aneurysm may demonstrate this finding. If a high-velocity neck is also identified, diagnostic confidence increases.
Connective Tissue Disorders
Patients with congenital connective tissue disorders such as Marfan syndrome or Ehlers-Danlos syndrome are at risk of dissection and aneurysm formation of the entire aorta, including the abdominal aorta. Abdominal aortic findings in this setting are often extensions of thoracic aortic dissections and aneurysms; therefore, any such finding on an abdominal CT should be further evaluated with dedicated imaging of the thoracic aorta ( Fig. 46-14 ). Finding dissection and aneurysm in the aorta of a young patient who was not otherwise involved in trauma should raise the suspicion of an underlying connective tissue disorder if the diagnosis has not been considered.

Pearls and Pitfalls
- ▪
Ultrasound and CTA are useful imaging modalities for abdominal aortic aneurysm disease; however, CTA is usually required for preprocedural planning.
- ▪
Mycotic aneurysms should be suspected in lesions with saccular morphology, rapid expansion, and periaortic inflammatory changes. Nuclear scintigraphy with indium-labeled leukocytes can be a helpful problem-solving tool.
- ▪
Aneurysms near suture margins should raise the suspicion of pseudoaneurysm.
- ▪
Dissection and aneurysm formation in a young individual may represent underlying collagen vascular disease. The examiner should look for patency of the true and false lumina and the branches arising from both.
Postoperative Aorta
Open and endovascular procedures of the abdominal aorta and branches have proliferated, and as a result, radiologists must be aware of the various imaging findings in the postoperative aorta. Knowing the radiologic appearance of bypass grafts, endovascular grafts, and combination procedures is helpful for understanding the expected imaging findings and therefore for rendering a helpful interpretation.
Aortobifemoral Bypass Graft
The traditional open operation for abdominal aortic aneurysms consists of aneurysm resection and repair with a tube graft or an aortobifemoral bypass graft. The sac is wrapped around the graft to provide a biologic barrier. The components of these grafts are not typically radiopaque and are therefore relatively easy to miss. Clues include subtle caliber differences at anastomotic margins and a double barrel appearance of the proximal iliac limbs as they course through the wrapped aneurysm sac ( Fig. 46-15 ).

Stent Graft Endovascular Aneurysm Repair
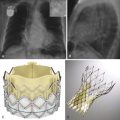
Endovascular stent grafts are easy to identify on CT because of the metallic stent struts. Various manufacturers for stent grafts exist, each with their own designs and configurations. Examiners should familiarize themselves with the CT cross-sectional appearance of these different products. In EVAR, because the aneurysm sac is excluded and not resected, the aneurysm sac is still visible. In fact, sizing the sac is a principal component of follow-up imaging to evaluate adequate exclusion of the aneurysm and the presence of ongoing pressurization of the aneurysm sac.
Key elements to evaluate on post-EVAR imaging are aneurysm sac size, possible endoleak, and possible stent graft migration or distortion ( Fig. 46-16 ). Although CTA is the primary diagnostic imaging modality for follow-up imaging after EVAR, the combination of ultrasound and radiography may also be performed if concern for an endoleak is minimal. Although duplex ultrasound has been shown to detect endoleaks, sensitivity is low, and evaluation for endoleak in the presence of an enlarging sac requires an additional study. The aneurysm sac size may stay stable or preferably decrease over time, but it should never increase after EVAR. If the sac size increases, it heralds the presence of an endoleak and persistent flow or pressurization within the aneurysm sac.

Five types of endoleak are recognized. Endoleak types 1 and 3 involve leakage at the junctions of the graft and native vessels or at the junction between graft components and are assessed carefully at the time of stent graft placement. When found, these endoleaks are corrected immediately. Endoleak type 4 is considered periprocedural secondary to systemic anticoagulation. Endoleak type 2 represents delayed opacification of the aneurysm sac from retrograde filling by branch vessels. Endoleak type 5 is controversial and is considered to represent ongoing pressurization of the aneurysm sac without a definite source of inflow. Of the endoleak types, endoleak type 2 is best evaluated on follow-up CT imaging with both arterial and delayed imaging. If contrast material is seen accumulating within the aneurysm sac, the examiner should look carefully for a feeding vessel. If the contrast material is accumulating posteriorly, it is often from a lumbar artery, and if the contrast material is accumulating anteriorly, it is often from a patent inferior mesenteric artery ( Fig. 46-17 ).