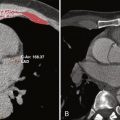
The use of magnetic resonance imaging (MRI) in cardiac diseases has been advancing rapidly, and this technique has become the gold standard for diagnosing many of the pathologic conditions encountered in clinical practice. MRI is an indispensable tool to aid in clinical decision making. Although traditional modalities such as single photon emission computed tomography (SPECT) and echocardiography continue to be the mainstays of cardiac imaging, MRI can provide data that are both complementary and uniquely distinct, thus allowing for insights into the disease process that until recently were not possible.
This chapter focuses on the ability of cardiac magnetic resonance (CMR) to aid in the management of both ischemic and nonischemic cardiomyopathies. Although much of this discussion highlights the ability of CMR to diagnose various myopathies, it also reviews the role of CMR as an important tool in various decision-making processes encountered on a daily basis by the clinician. Such instances include deciding whether to pursue revascularization in ischemic cardiomyopathies and determining when it is appropriate to recommend implantation of an implantable cardioverter-defibrillator (ICD) in the myopathic heart.
A clinical vignette is offered as an introduction to each topic discussed. The hope is that such a format will highlight the practical implications of the current data available on the use of CMR in a particular pathologic process. Although the amount and quality of supportive data may vary for each clinical instance, the data reflect the “real world” challenges that clinicians face when treating patients.
Ischemic Cardiomyopathy: When to Revascularize?
Case 1
A 70-year-old male patient with a history of diabetes, hypertension, and elevated cholesterol and a prior history of a distant myocardial infarction undergoes an evaluation for gradually progressive dyspnea on exertion. An echocardiogram shows a mildly dilated left ventricle and an ejection fraction of 38% with regional wall motion abnormalities. Exercise treadmill testing with SPECT imaging is performed, and the patient is able to exercise for 5 minutes of Bruce protocol (see Chapter 5 for details of Bruce protocol). No changes are noted on the electrocardiogram (ECG), and the patient has mostly fixed defects in the anterior and lateral walls with “mild reversibility.” He is already taking a beta-blocker, a statin, aspirin, and an angiotensin-converting enzyme (ACE) inhibitor as part of his medical regimen.
Should the patient undergo cardiac catheterization even though the clinician knows that this patient will have significant coronary disease, given that noninvasive imaging seems to indicate mostly scarred myocardium? Would any benefit result from revascularization with the present data from the stress test and echocardiogram? Would an invasive strategy provide any benefit, given that the patient is already following a good medical regimen?
Importance of Viable Myocardium
The clinician should establish whether any chance exists that the patient has viable myocardium that will benefit from restoration of blood flow. In patients with an area of previously infarcted myocardium, the tissue is often a heterogeneous mixture of both scar and noncontractile but still surviving myofibers. Although the tissue has reduced perfusion from the ischemic substrate, residual metabolic function is present. This dysfunctional but viable tissue in the setting of chronic coronary disease has been classified as hibernating myocardium. In patients with viable myocardium, restoration of blood flow through revascularization improves survival as compared with patients who are treated medically ( Fig. 21-1 ).

This mortality benefit occurs even without an improvement in left ventricular (LV) function. Studies also have shown that attempts to revascularize nonviable tissue provide no survival benefit and stress the need to be sure that the diagnostic imaging has high sensitivity and specificity. However, patients with severely dilated hearts tend to fare worse than do patients with smaller LV volumes. This finding may reflect the chronicity of the ischemia or the extent of adverse remodeling. Even in patients with demonstrable viable tissue, revascularization may not be possible, especially in those with prior coronary artery bypass grafting or diabetic patients with poor target vessels. Despite areas of viable myocardium, these patients are treated medically, with the subsequent risk of higher mortality. Not surprisingly, in small, nonrandomized studies, sudden cardiac death was the leading cause of mortality in patients with hibernating myocardium who were treated medically.
Role of Cardiac Magnetic Resonance in the Search for Viable Tissue
Is a superior method available to assess whether any of the tissue is viable? Most clinicians agree that all the traditional methods (dobutamine MRI, contrast-enhanced MRI, SPECT with thallium-201, SPECT with technetium-99m tracers, positron emission tomography/SPECT with fluorodeoxyglucose) provide the data necessary to assess whether and to what extent viable tissue is present. Few data are available that compare noninvasive methods “head to head” to determine which is superior. CMR is able to detect viable tissue by measuring end-diastolic wall thickness, evaluate contractile reserve, and detect the degree of transmurality of scar tissue. The ability to identify the presence of transmural scar (involving the entire thickness of myocardium) is important because this finding suggests that functional recovery will not improve even with restoration of blood flow. Earlier studies validated the use of MRI in the assessment of viable myocardium, and MRI can distinguish infarcts with variable degrees of nontransmural scar from transmural scars. Generally speaking, myocardium with a scar (delayed hyperenhancement) that occupies less than 50% of the wall thickness is likely to improve in function after revascularization.
Data suggest that the sensitivity and specificity of regional wall improvement as predicted by CMR are 82% and 67%, respectively. Studies suggest that CMR carries important prognostic information for general cardiovascular outcomes. An additional benefit of CMR is the absence of radiation, although caution must be taken if gadolinium is used in patients with severe renal insufficiency. In general, most clinicians agree that at least 25% of the left ventricle should be “viable” to pursue revascularization.
Case 1 Continued
The patient has diabetes and a depressed ejection fraction. If the patient has significant coronary disease (especially left anterior descending coronary artery disease) that can be revascularized, the patient’s life can be prolonged. The use of CMR may be beneficial in this situation if the actual amount of viable tissue is near this cutoff, as may be suggested by the initial SPECT imaging (although it was not a viability study). Cardiac catheterization would be reasonable to establish coronary disease as the cause. Revascularization can then be performed if viable tissue is indeed present.
Ischemic Cardiomyopathy and the Risk of Sudden Cardiac Death
Case 2
A 69-year-old male patient with a history of coronary artery disease with prior coronary artery bypass grafting 10 years earlier and known ischemic cardiomyopathy presents to his cardiologist for a yearly checkup. The patient feels well and walks regularly 5 days a week for 20 minutes at a time. He has been closely followed by his cardiologist, and his last echocardiogram a year ago was remarkable for a mildly dilated left ventricle with an ejection fraction of 40% without significant valvular abnormalities. The patient’s medication regimen includes aspirin, a beta-blocker, an ACE inhibitor, a statin, and a low-dose diuretic. His ECG shows sinus rhythm at 64 beats/minute with occasional premature ventricular contractions (PVCs), an old anterior wall myocardial infarction, and a QRS complex duration of 96 msec. He has New York Heart Association (NYHA) class II congestive heart failure (CHF), and a 24-hour Holter monitor shows occasional PVCs with couplets, but no triplets. A repeat echocardiogram shows an ejection fraction of 34%. Repeat stress testing with perfusion imaging results in his exercising for 9 minutes of Bruce protocol (10 metabolic equivalents [METS]) without ECG abnormalities. Perfusion imaging shows scar in the anterior apical region without reversible ischemia with a calculated ejection fraction of 38%. After the data are obtained, the patient’s cardiologist opens the conversation about the role of an ICD. The patient feels well and desires more information and data about whether he really needs one. The patient’s internist suggests cardiac MRI to obtain a “true” ejection fraction.
Sudden Cardiac Death: Can Cardiac Magnetic Resonance Provide Useful Prognostic Information?
Sudden cardiac death, the leading cause of cardiovascular mortality in the United States, claims approximately 400,000 lives each year. Patients with a history of coronary artery disease, previous myocardial infarction, and depressed LV function are at increased risk for ventricular arrhythmias, which are responsible for most cases of this syndrome.
Based on data from the Sudden Cardiac Death from Heart Failure Trial (SCD-HeFT) and the Multicenter Automatic Defibrillator Implantation Trial (MADIT II), the current standard of care supports the implantation of an ICD in the patient in this case study. The SCD-HeFT enrolled patients with ischemic or nonischemic cardiomyopathy with an ejection fraction of up to 35% and class II or III NYHA, whereas the MADIT II enrolled patients with ischemic cardiomyopathy with an ejection fraction of up to 30% and NHYA class I, II, and III CHF. Further examination of the two trials found that approximately 20% of implanted ICDs had fired appropriately during 4 years of follow-up, thus subjecting most patients to the morbidity of having implanted hardware without receiving benefit. Although ejection fraction is a strong predictor of ventricular tachyarrhythmias, it is relatively nonspecific, and other clinical characteristics are needed to help stratify risk in these patients appropriately.
Because of the lack of a satisfactory way to stratify these patients, the search for a diagnostic test that could serve as a noninvasive means of identifying patients at risk for sudden cardiac death has rapidly evolved. Exercise treadmill testing and myocardial perfusion imaging have provided important prognostic information for patients at risk for cardiovascular morbidity and mortality, but not specifically for arrhythmogenic death.
Mechanism of Ventricular Tachycardia
To help identify patients at risk for sudden death, an understanding of the basic mechanism of ventricular tachycardia (VT) in the patient with coronary artery disease is critical. Earlier work in animal and human pathologic specimens showed that both a substrate and a trigger are required for the initiation and propagation of the reentry circuit. The trigger that initiates the arrhythmia may result from multiple factors such as ischemia, changes in autonomic tone, and neurohormonal and metabolic influences. The anatomic substrate is scar tissue around which propagation exhibits slow conduction and thus allows for maintenance of reentry circuits. The surviving myocardial fibers surrounding and interspersed within the infarcted region provide the electrical heterogeneity that is required for differences in conduction velocity, functional block, and alterations in cell-to-cell coupling.
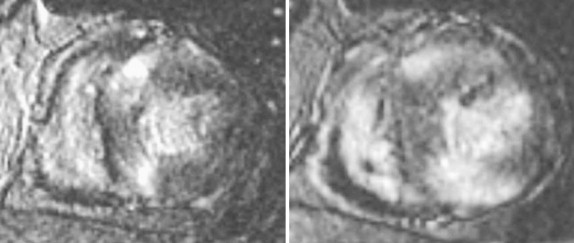
The elucidation of this mechanism was demonstrated elegantly in explanted human hearts studied with high-resolution mapping ( Fig. 21-2 ). In these hearts that had sustained VT, mapping localized slow conduction that is critical for reentry along a complex network of surviving myocytes interspersed in and around the scar. These findings helped to complement earlier work in animal models that demonstrated the presence of a circuit in the “border zone” of tissue made of scar and surviving myocardium.

Detecting Scar and Ventricular Tachyarrhythmias: Is Quantification of Scar Enough?
Knowing that the presence of scar or fibrosis could represent a potential substrate for ventricular tachyarrhythmias, investigators have sought to determine whether prognostic information can be obtained using CMR in various myopathic processes, including hypertrophic cardiomyopathy (HCM) and ischemic and nonischemic cardiomyopathies.
Earlier data suggested that total cardiac enzyme released during a myocardial infarction is a predictor of future cardiovascular outcomes. The absolute quantity of necrosed tissue may therefore be reflected by the extent of scarred myocardium. The extent of scar tissue, as determined by stress testing with myocardial perfusion (technetium) imaging, has been demonstrated to be an important predictor of death and ventricular arrhythmias. In 349 patients with ischemic cardiomyopathy and a depressed ejection fraction, the degree of quantifiable scar as determined by CMR was a predictor of death or the need for cardiac transplantation. In addition, this study found that female gender with scar was also a positive predictor of cardiac events. Similarly, delayed enhanced MRI (DE-MRI) performed in 231 patients with prior healed myocardial infarctions demonstrated that infarct size was a better predictor of long-term mortality than was LV ejection fraction.
Although the ability to quantify scar accurately in patients with coronary artery disease may be useful to reflect the size of a prior myocardial infarction accurately, whether this information will be useful to predict who is likely to develop ventricular tachyarrhythmias, as opposed to mortality resulting from CHF or myocardial infarction, is not clear. One small study found that infarct mass, as determined by DE-MRI, was shown to be a better predictor than LV ejection fraction of inducible monomorphic VT at electrophysiology study in patients ( n = 48) with a history of coronary artery disease.
Investigators had hoped that the DETERMINE study would determine whether infarct mass alone could appropriately stratify risk in patients. This study was to test the hypothesis that patients with an infarct size of at least 10% randomized to ICD and medical therapy would have improved survival as compared with those randomized to medical therapy alone. Patients with coronary disease and an ejection fraction between 35% and 50% (or patients with an ejection fraction of 30% to 35% and NYHA class I heart failure without a history of ventricular tachyarrhythmias) would have undergone CMR imaging to determine the infarct size. Unfortunately, the trial was stopped by the sponsor. As of now, the investigators have no future plans to move forward, and so the answer to this question remains unknown.
Characterizing the Border Zone: The Intersection of Scar and Viable Tissue
Although the presence of scar is critical in forming the basis of the substrate for ventricular tachyarrhythmias, a considerable effort has been made to try to “visualize” the interface between scar and surviving tissue. Attempts to characterize scar and the surrounding border zone in patients with a prior myocardial infarction have been made using CMR. Furthermore, initial reports seem to yield promising results regarding prognostic information in potentially high-risk patients.
An initial report characterized the mechanical properties of LV wall segments containing different degrees of scar tissue and the location of these segments from the interface between infarcted and noninfarcted myocardial tissue. A total of 46 patients underwent electrophysiologic testing before subsequent implantation of an ICD for primary prevention of sudden cardiac death. Patients with inducible monomorphic VT during electrophysiology study were more likely to have more infracted and border zone segments as compared with patients whose VT was noninducible. Furthermore, patients with inducible monomorphic VT were more likely to have border zones segments with greater systolic contractility as compared with patients with noninducible VT. These findings suggested that “enhanced” border zone function as determined by DE-MRI may be a marker of inducible monomorphic VT. This characterization of the mixture of viable and nonviable tissue (tissue heterogeneity) and how it relates to arrhythmogenesis further strengthened the potential prognostic capabilities of DE-MRI.
More recently, Roes et al examined whether infarct tissue heterogeneity could serve as a predictor of spontaneous ventricular tachyarrhythmias. Ninety-one patients with a prior myocardial infarction underwent DE-MRI before implantation of an ICD. After a median follow-up period of 8.5 months, 18 patients had received appropriate ICD therapy. Infarct tissue heterogeneity was a better predictor of appropriate ICD therapy as compared with LV function, LV volume, and total infarct size.
Specific characteristics of the interface between scar and surviving tissue are likely crucial determinants of whether a substrate exists. Although the hope is that CMR can identify and characterize the extent of this “gray” zone, several limitations must first be overcome. Certain technical considerations are important when assessing the characteristics of the periinfarct territory known as the border zone. This mixture of viable and nonviable tissue alters signal intensity because partial volume effects produce intermediate signal intensities along the border zone. The quality of the study is critical; proper adjustment of the inversion time is crucial in interpreting the images. Manually adjusting the inversion time to null signal from normal myocardium is necessary in each patient to optimize contrast between normal myocardium (black) and scar (white).
Case 2 Continued
CMR could provide the clinician and the patient with several pieces of relevant data. It could provide an ejection fraction that is likely to be the most accurate. In addition, it could quantify the extent and characteristics of scar that is present, and this could also help predict the likelihood of cardiovascular morbidity and mortality for this patient given the earlier (albeit limited) studies.
Hypertrophic Cardiomyopathy
Case 3
A 24-year-old white male patient without any significant medical history presents after a syncopal episode while food shopping. He lost consciousness, fell on his forehead, and required eight stitches to close a laceration. The patient has no recollection of the event. He has never had syncope or near syncope in the past and was active in high school sports. He has had “a few” palpitations once in a while that have lasted for seconds but has not thought anything of it. This patient is not taking any medications and has never used illegal drugs. He has no family history of sudden cardiac death. Physical examination is unremarkable, without any murmurs; laboratory study results are normal. An ECG shows sinus rhythm with nonspecific T-wave abnormalities. An echocardiogram shows normal biventricular size and function without significant valvular abnormalities. The interventricular septum is measured at 12 mm, as compared with 9 mm in other parts of the left ventricle. An exercise stress test is unremarkable; the patient exercises for 12 minutes of Bruce protocol with a normal blood pressure response and no ECG changes. A 24-hour Holter monitor shows rare PVCs.
The patient’s cardiologist is concerned about the mild septal hypertrophy and brings up the possibility of HCM. The patient himself wonders whether this could have been his first episode of vasovagal syncope. The cardiologist refers him to an electrophysiologist and asks whether an electrophysiology study would help. The electrophysiologist decides to order a cardiac MRI. Is this reasonable?
Traditional Methods for Risk Stratification of Sudden Cardiac Death
The diagnosis of HCM can sometimes be challenging, especially in patients who are high-endurance athletes. Once the diagnosis is entertained, the concern for sudden cardiac death must be considered, and the patient must be assessed appropriately. The mechanism of sudden cardiac death in these patients has been shown to be mainly VT or ventricular fibrillation. Many patients present with sudden cardiac death, especially during an episode of exertion. At present, risk stratification of sudden cardiac death includes a family history of sudden death, a history of syncope, nonsustained VT (NSVT) on Holter monitoring, abnormal blood pressure response during exercise stress testing, and LV hypertrophy of 30 mm or greater. However, data suggest that many patients experience an event with only one risk factor. Long-term follow-up in a large cohort suggested that appropriate ICD discharges are just as likely in patients with one risk factor as they are in those with three or more risk factors. As a result, recommendations about prophylactic implantation of a defibrillator are difficult in patients who may have only one such risk factor. A careful assessment must be made on an individual basis.
Cardiac Magnetic Resonance and Delayed Enhancement in Hypertrophic Obstructive Cardiomyopathy
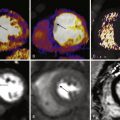
The pattern of scarring in HCM that has been detected with CMR usually does not occur in the territory of the epicardial coronary arteries. Instead, typical patterns of fibrosis are seen on CMR. Unlike ischemic scars, which are always subendocardial with varying degrees of transmurality, the fibrosis in HCM is typically midmyocardial and shows sparing of the subendocardial myocardium. The patches of fibrosis are not confined to a coronary artery territory. Several studies have reported that fibrosis of the myocardium at the insertion sites of the right ventricle into the ventricular septum is characteristic of HCM ( Fig. 21-3 ). Studies have also shown that the presence of scar and fibrosis in patients can be reliably detected using CMR, with good histologic correlation of the scar with areas of delayed enhancement. More important, the use of this imaging modality may have prognostic significance in these patients.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree