Clinical Presentation
A sixty-five-year old white female with severe chronic obstructive pulmonary disease (COPD), hypertension, hyperlipidemia, and a 3-week history of mild-to-moderate intensity low back pain and abdominal pain. The patient has smoked one pack of cigarettes per day for the past 30 years. Physical examination reveals a pulsatile abdominal “mass.”
Imaging Presentation
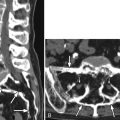
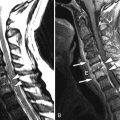
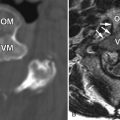
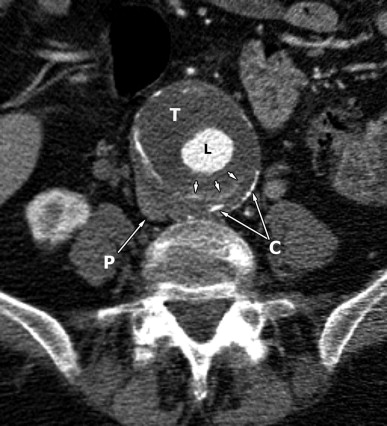
Computed tomography (CT) revealed a 7-cm diameter aneurysm of the infrarenal abdominal aorta ( Figs. 6-1 to 6-4 ) . The thoracic aorta was markedly tortuous. The patient underwent arteriography, which documented the following findings: Aortic neck diameter adjacent to aneurysm—33 mm; maximal aortic aneurysm lumen diameter—58 mm; terminal aortic diameter—42 mm.



Clinical Course
The patient was not considered a surgical candidate for open aortic aneurysm repair because of her severe COPD and therefore underwent endovascular repair of the abdominal aortic aneurysm with insertion of an aortoiliac stent graft. The procedure was complicated by inadvertent rupture of the left common iliac artery, requiring interposition of an iliofemoral graft. The patient later developed an incisional hernia. She developed intermittent urinary tract infections requiring antibiotic therapy. A 2-year follow-up CT scan revealed diminished size of the aneurysm sac, measuring 5.9 × 4.4 cm in transverse diameter. Some calcium was observed in the intramural thrombus in the aneurysm sac, but no endoleak was observed.
Discussion
An abdominal aortic aneurysm (AAA) is defined as an ectatic region of the aorta exceeding twice the normal diameter (approximately 3 cm). AAA should always be considered in an elderly patient with low back pain. However, abdominal aortic aneurysms are an uncommon cause of isolated low back pain but can cause severe back pain in the setting of a rupturing or dissecting aortic aneurysm. Aortic aneurysms rarely occur before age 50 but are found in 2% to 4% of the population after the age of 50. For each decade beyond 65 years, the prevalence of AAA increases by 2% to 4% per decade. Average age of diagnosis of AAA is between 65 and 70 years old. Abdominal aortic aneurysms are more common in men.
The presence of coronary artery disease and peripheral vascular disease, as well as a family history of AAA, are strong risk factors. Cigarette smoking is a particularly strong risk factor for the development of abdominal aortic aneurysms. Smoking is believed to cause degradation in elastin within arterial walls. Ruptured AAA and aortic dissection (AD) are significant causes of mortality in persons over age 65 years, accounting for nearly 1% of deaths. Rupture of an AAA and aortic dissection account for an estimated 15,000 deaths annually in the United States. Aortic dissection and ruptured aortic aneurysm are life-threatening conditions that should always be considered in the diagnosis of back pain. The mortality for untreated aortic dissection reportedly increases at a rate of 1% to 3% per hour during the first 24 hours of illness. For patients who have a rupturing AAA, the overall 30-day survival is reported to be as low as 11% despite rapid surgical intervention.
Aortic Dissection
The incidence of aortic dissection (AD) is highest between ages 50 and 70 with a 5:1 male to female preponderance. The diagnosis is missed initially in up to 40% of these patients, because they often lack the classic presentation, that is, a sharp, knifelike, tearing pain; 80% of aortic dissection patients are hypertensive. Other risk factors include smoking, hyperlipidemia, bicuspid aortic valve, coarctation of the aorta, decelerating trauma, cocaine usage, and inflammatory conditions of the aorta. Disorders that damage the aortic wall or are associated with an elastic deficiency in arterial walls predispose to aortic dissection; these include collagen vascular disorders, and Marfan, Ehlers-Danlos, Turner, and Noonan syndromes. Patients with aortic dissection may present with neurologic findings including cerebral and spinal cord ischemia. Quadriplegia, paraplegia, or unilateral paresthesias may also occur. Aortic dissection is caused by an intimal tear allowing luminal blood to extend into the medial layer of the aortic wall, forming a false channel or lumen within the media.
Ruptured Aortic Aneurysm
The classic clinical triad of hypotension, abdominal pain, and a palatial mass occurs in less than 50% of patients who have a rupturing AAA. Patients with rupturing AAA more commonly present with back pain, left lower-quadrant pain, flank pain, syncope, or lower-extremity paresthesias and therefore initially may be misdiagnosed as having renal colic or diverticulitis. The mortality rate in acute aortic rupture is approximately 60%, whereas chronic ruptures may evolve over months with chronic low back pain. Aortic ruptures may occur at a relatively smaller diameter in females. Observational evidence suggests that the presence of intraluminal thrombus may have a protective effect, because there tends to be relatively less intraluminal thrombus in ruptured aneurysms compared with nonruptured aneurysms.
Inflammatory aneurysm is an older term used to describe a noninfectious aneurysm subtype that ruptures slowly producing an inflammatory response and adhesions in the retroperitoneal and prevertebral tissues surrounding the aorta. The etiology of inflammatory aneurysms is not always known, but may be related to periaortic retroperitoneal fibrosis and possibly may be caused by autoimmune disease, such as rheumatoid arthritis, systemic lupus erythematosus, or giant cell arteritis.
Infectious (mycotic) aneurysms are uncommon, representing 1% to 2% of aortic aneurysms, but have a significantly higher incidence of rupture. Hematogenous seeding of the aorta usually occurs because of septicemia, most commonly due to endocarditis. Most infected aortas occur in the thoracic or suprarenal abdominal aorta. Infected aortic aneurysms rarely cause infection in the adjacent spine. Infections usually produce irregular vertebral bone destruction. Discovertebral osteomyelitis or renal or psoas muscle infections may rarely cause infection of the aorta. Tuberculous vertebral osteomyelitis and associated psoas muscle infections may extend to involve the aorta.
Chronic contained ruptures of the aorta are relatively rare, occurring in approximately 2% of aortic aneurysms going to surgery. Chronic contained ruptures (also called “leaking” or “sealed” ruptures) may incite a perianeurysmal inflammatory and fibrotic response, and these slowly enlarging aneurysms may erode the adjacent vertebrae, which can be responsible for the low back pain in these patients. Chronic contained ruptures of the aorta tend to cause smooth erosion of the vertebrae. Support from the adjacent vertebrae may provide a tamponade effect for the chronic contained rupturing aneurysm.
Penetrating atherosclerotic ulcers are found in 2% to 10% of patients examined for significant aortic abnormalities and may present with symptoms similar to aortic dissection, that is, chest pain and back pain. Penetrating aortic ulcers may result in hematoma formation in the aortic media but are not believed to be a common cause of aortic dissection. They can result in false aneurysm formation and, rarely, transmural rupture of the aorta.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree