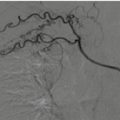
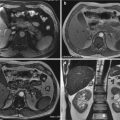
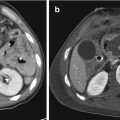
Fig. 22.1
Liver CECT scan before and after angioembolization, arterial phase: (a) evidence of active bleeding (white arrow) and subcapsular fluid collection (white arrowhead); (b) presence of metallic coils (black arrowhead) and absence of bleeding
In literature several authors highlighted good results with treatment of bleeding even in haemodynamically unstable patients: in particular Monnin et al., with a multidisciplinary approach including embolization, has achieved a 93% success also with haemodynamically unstable patients [14]. Furthermore, in trauma patients who respond to resuscitation treatment, the guidelines of the Eastern Association for the Surgery of Trauma (EAST) in first instance recommend (level 2) arterial embolization, possibly in addition to surgery [15]. Indications for conventional hepatic angiography include active contrast extravasation identified by CECT, evidence of ongoing bleeding despite conservative resuscitative measures, haemobilia and control of continued haemorrhage after surgery. Active bleeding is the leading indication for interventional radiology procedures, in particular as a valid support to the NOM; hepatic angioembolization is usually performed in a dedicated angiographic suite and starts with a retrograde common femoral approach: the goal is to selectively catheterize the hepatic artery and after superselective catheterization reaching up to the site of vascular disease (extravasation, pseudoaneurysm or arteriovenous fistula) to embolize using coils or embolic agents (liquid agents such as Glue and Onyx, gelfoam) (Fig. 22.2).


Fig. 22.2
Angioembolization of hepatic bleeding arteries (black arrowhead) by the selective catheterism of the celiac trunk using a Simmons 1 shaped macrocatheter (white arrow), super selective microcatheterism of the bleeding artery (black arrows) and microcoils deployment (white arrowheads)
The most common vascular catheter used for the celiac trunk is Cobra or Simmons shaped; the external size of microcatheters used for superselective catheterization usually has a range from 2.0 to 2.8 French (3 Fr = 1 mm). In case of pre-procedural CT with extensive liver injury, some authors suggest to release embolizing agents such as gelfoam or Spongostan from the hepatic artery of affected lobe even in the absence of angiographic evidence of active bleeding: this however may help in achieving haemostasis and haemodynamic stabilization of the patient; however, it is controversial [16]. In the setting of trauma, the technical success rate of hepatic angioembolization ranges from 88% to 100%, but in cases following surgical packing owing to distorted anatomy and manipulation, technical success rate drops to 60–70% [14]. Usually technical difficulties are related to vascular tortuosity, artery stenosis, most commonly of the celiac trunk, and vessel spasm. In case of complex vascular anatomy, it may help to use smaller size and more navigable microcatheters; similarly, in case of vasospasm, the use of microcatheter and delicate catheter manipulation will help to reach the site of hepatic injury [16]. Lee et al. reported 11 cases of incomplete embolization: ten of these cases were secondary to a persistent contrast blush without an identifiable vessel or a blush supplied by multiple collaterals that could not be embolized, and one reported failure secondary to a stenotic celiac artery [17]. Both Lee et al. and Hagiwara et al. reported failures of NOM despite technical success of angioembolization [18]. Often, the failure of conservative management despite successful angioembolization is related to venous bleeding, that can be difficult to identify during angiography and even harder to treat.
Late liver bleeding is an uncommon (3% of liver trauma) possible complication of NOM [19]. The number of complications is higher in patients with higher degree liver injuries; the most common complications of a hepatic angioembolization include hepatic necrosis, abscess formation and non-target embolization [20]. Due to its vascular conformation, the liver receives constant inflow from both portal and arterial venous vessels. However, despite this robust dual supply, the combined insult of trauma and embolization has been shown to cause significant hepatic necrosis that ranged from 0 to 42% with a weighted mean rate of 15% and was associated with longer hospital stay and increased transfusion requirement [8]. It is evident that liver trauma and subsequent liver devascularization play a major role in hepatic necrosis compared to angioembolization, in particular with a superselective embolization. Green et al. reported that a major degree of arterial selectivity during embolization was generally related to a lower necrosis rate [8].
The next two common complications are abscess formation and bile leak/biloma [21, 22]. These complications are not clearly related to angioembolization and, in literature, they are reported both in operative and non-operative management of hepatic trauma [23, 24]. Probably, hepatic injury and death of a large number of hepatocytes together create a combined state of parenchymal flogosis and tissue necrosis, increasing the risk of abscess formation independently from angioembolization.
Even in the management of complications, interventional radiology through the positioning of percutaneous drainage on abscess sites plays a key role. In a multidisciplinary approach, today the conservative management in patients with liver injury appears a successful algorithm in order to reduce mortality and morbidity rate: angiography and angioembolization are essential components of successful NOM of hepatic trauma patients, as well as a critical component of haemorrhage control following laparotomy [25, 26]. In particular, despite there are no consensus guidelines on appropriate patient selection criteria for those who would benefit from angiography and angioembolization, several articles have suggested that early angiography and embolization improve outcomes in patients with high-grade hepatic injuries [12, 26–30].
In summary, hepatic angioembolization is an effective and important component in the management of traumatic hepatic haemorrhage, also in an operative or non-operative management. Hepatic necrosis is the most important complication and can occur following embolization, but this risk can be safely managed by medical therapy without serious sequelae.
Today interventional radiologists, as real “playmakers” of the multidisciplinary team, play a central role in the treatment of hepatic trauma.
22.4 Aorta
Traumatic aortic injury, a consequence of penetrating injuries or blunt trauma, is a life-threatening condition, which requires prompt diagnosis and management: the vast majority of patients die before they can receive medical care; furthermore, the mortality rate for patients with aortic rupture who survive until they reach the hospital is estimated to be 41–50% [31]. Traumatic aortic injury commonly occurs at the sites of aortic tethering that is the aortic root, the isthmus and at the diaphragmatic hiatus; although it is rare in the abdomen, the most common site of injury is the infrarenal abdominal aorta [32].
Abdominal vascular injuries can be caused by penetrating or blunt trauma. Blunt Abdominal Aortic Injuries (BAAIs) are caused by traffic accidents commonly referred to as the “seat belt aorta” [33, 34]. These injuries are rare in the current reported world literature because the thoracic aorta is fairly well protected by the bony thoracic cage [35]. Starnes et al. proposed a CT-based management classification system for BAAI [36]: he classified BAAI into 4 types based on aortic external contour abnormality, intimal tear and contrast extravasation. Most patients with BAAI rarely reach the hospital alive and those who do have a reported 24% mortality. Most abdominal aortic injuries have been repaired via an open surgical approach, with endovascular stent graft as an alternative. Despite significant improvements in critical care support, non-invasive diagnosis, anaesthesia and surgery over the last few decades, the conventional open surgical repair of an aortic rupture still carries a significant risk of serious complications and mortality, and in patients with concomitant injuries and comorbidities is associated with high morbidity and mortality [37–39]. Therefore, endovascular repair techniques have emerged as a promising alternative in these patients [40]. Several case reports and case series evaluating the technical feasibility and safety of endovascular treatment for ruptured abdominal aortic aneurysm (rAAA) or a thoracic aortic injury (TAI) suggest that between 40% and 80% of rAAAs are suitable for endovascular aortic repair (EVAR), with a perioperative mortality rate of 10%–29% for endovascular repair of rAAA [41–44].
Both conventional open surgical and endovascular treatment options for aortic ruptures are available in referral centres; however, the transferring of patients who need emergency treatment to a referral centre is usually not possible. Therefore, the widespread use of endovascular techniques is important for the rapid and adequate treatment of these patients [45]. A key role in the success of endovascular approach is an accurate patient selection by an early Angio-CT examination assessing the feasibility of the endovascular procedure (aneurysm neck, iliac axes involvement, etc.) as well as the evaluation of haemodynamic and vital signs; so, an optimal patient selection for EVAR requires a multidisciplinary approach including interventional radiologists, vascular surgeons, emergency doctors and anaesthetists.
In conclusion, in cases of abdominal aorta ruptures from blunt trauma, endovascular aortic repair is evolving and offers the potential to improve mortality rates in acute aortic injuries: therefore, interventional radiology can play a crucial role in trauma centres.
22.5 Spleen
The spleen is the second abdominal organ most commonly involved in closed abdominal trauma after the liver. Splenic injuries, occurring in 32% of abdominal injuries, most often are observed in blunt abdominal trauma such as in traffic accidents, assaults, fall from height and sports. Spleen damage is a serious and fearful consequence of abdominal trauma; as it is a highly vascularized organ, splenic injuries can result in prolonged bleeding with the risk of a threat to the life of the patient if not recognized and treated correctly.
In addition to the immediate danger caused by intraperitoneal bleeding with haemorrhagic shock potential, delayed bleeding may occur in capsular lesions or post-traumatic vascular lesions (artery venous fistula, pseudoaneurysms), that may cause severe bleeding even after discharge from the hospital [46].
The method of choice for rapid evaluation of the abdomen to early identify the presence of abdominal free fluid is the Focused Abdominal Sonography for Trauma (FAST), that can be performed simultaneously with resuscitation efforts during the initial trauma management. For this reason it is also useful in haemodynamically unstable patients. However, FAST has a low sensitivity for detecting and grading splenic injuries and is unable to detect the presence of active haemorrhage.
CT is the imaging modality of choice for haemodynamically stable patients with blunt abdominal trauma [47], being the most accurate test to assess the grade of injury, and it can give a relatively accurate evaluation of the volume of haemoperitoneum. It can also detect the presence and location of active arterial bleeding as well as the presence of pseudoaneurysms or arteriovenous fistulas in the spleen (Fig. 22.3).


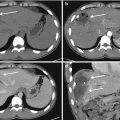
Fig. 22.3
CECT scan, arterial (a and b) and portal (c and d) phases: splenic subcapsular laceration (white arrows) and parenchymal haematoma (black arrowheads) with pseudoaneurysm (white arrowheads); (e) arterial phase 3D Volume Rendering
Splenic injuries can be radiologically classified using The American Association for the Surgery of Trauma (AAST) grading system (see table in Chap. 16) [48, 49]. This grading system is based on the anatomic extension of disruption of the spleen, as shown on CECT scans or during laparotomy. However, this grading system is not reliable for the prediction of the outcome of splenic injuries and not decisive about whether surgery or conservative treatment should be applied.
Historically a large proportion of patients with bleeding splenic trauma were subjected to emergency splenectomy to avoid the risk of fatal haemorrhage. However, 45–49% of patients undergoing surgery for splenic lesions present post-operative complications including pneumonia, bacteraemia, urinary tract infections and abscesses. Surgical splenectomy is associated with a mortality of 8–10% [50]. NOM of blunt splenic injury by clinical observation alone has a reported failure rate of 34–52% with these patients often requiring subsequent splenectomy.
In 1981, Sclafani published the first case report of arterial embolization for traumatic splenic injury as an alternative to surgery. The development of advanced endovascular interventional techniques has led to greater consideration of NOM for haemodynamically stable patients. Splenic artery angiography and either selective embolization for active haemorrhage or proximal splenic arterial embolization can be used to reduce the risk of delayed haemorrhage. Worldwide the majority of trauma centres now prefer NOM in haemodynamically stable patients with blunt splenic injury. As a result, splenic artery embolization (SAE) is increasingly performed reducing the need for operative intervention [51]. Wait-and-see approach has been reported to have a failure rate as high as 34%; the rate is even higher among patients with high-grade splenic injury (AAST grade III–V) [52–55].
Splenic arterial catheterization is commonly performed using common femoral artery access. Placement of a 5–6 French introducer sheath is sufficient in most cases. Selective angiography of splenic artery should always be performed to evaluate arterial injury type and extension. Diagnostic series of the splenic artery can be obtained using a macrocatheter, but for selective catheterization of the splenic artery branches coaxial microcatheters and micro-guidewires may be required. Technique and materials used for embolization depend on anatomical considerations, the haemodynamic situation of the patient, and the type and distribution of vascular injuries. Coils are usually the embolic agents of choice, and gelfoam may be used in some critical situations requiring quick and massive arterial occlusion (Fig. 22.4). Occasionally, the use of an Amplatzer vascular plug may be useful [56].


Fig. 22.4
Angioembolization procedure: (a) diagnostic angiogram showing a peripheral vascular lesion of the spleen (white arrow), (b) microcatheterism of the bleeding vessel and coils positioning (white arrowheads), (c and d) post-embolization angiograms showing complete haemostasis
Haemostasis after coil embolization usually occurs as a result of coil-induced thrombosis rather than mechanical occlusion of the lumen by the coil; therefore, this technique works best when the coagulation profile of the patient is normal or only mildly abnormal. In case of serious clotting disturbances, addition of another embolic agent, such as gelfoam, is indicated. Gelfoam is a sterile gelatine sponge intended for use as a temporary intravascular embolic material and it can be used in the shape of a “torpedo” or as pledgets. The major advantage of coils compared with gelfoam is the ability to improve permanent embolization, which is most desirable in treating vascular injuries. In fact, temporary occlusive agents such as gelfoam seem to have a higher failure rate (50%) compared with coil embolization (23%) [57].
There are two angiographic techniques to achieve splenic haemostasis [58]: Proximal Splenic Artery Embolization (PSAE) and selective distal splenic embolization. Proximal embolization of the splenic artery is the surgical equivalent of the arterial ligature and is based on the concept that decreasing the flow and splenic pressure the bleeding should consensually stop; rescue of the organ is ensured by sufficient perfusion through collateral gastric and pancreatic arteries. Coils are generally used for proximal embolization and, in some specific situations, also Amplatzer vascular plugs can be deployed to achieve PSAE. Proximal embolization is used in cases of widespread spleen bleeding, i.e. when there are multiple focal bleeds, and/or when the haemodynamic conditions of the patient require fast treatment; moreover, it can be performed when the splenic artery tortuosity prevents selective distal embolization. It is also used in those situations where the haemorrhage site is not identifiable at angiography but the clinical situation of the patient suggests active splenic bleeding. A potential disadvantage of proximal embolization of splenic artery may be that in the event of a bleeding recurrence it may be difficult to repeat the embolization as the catheterism of splenic artery could not be possible anymore.
Selective distal embolization only concerns damaged arterial blood vessels, usually in their distal part within the splenic parenchyma; generally, this type of embolization requires the use of a microcatheter. A potential complication of selective distal embolism is the possibility of splenic infarction with superinfection and abscess formation, which rarely lead to clinical consequences and are generally treated percutaneously by the positioning of a drainage catheter.
Follow-up is usually clinical but in some selected cases, when distal embolization is performed to exclude some unstable lesions of the splenic artery as pseudoaneurysms or arteriovenous fistulas, a CECT scan could be repeated to assess the complete exclusion of the treated lesion (Fig. 22.5).
In conclusion, splenic artery embolization is a safe and effective procedure for the NOM of splenic trauma in haemodynamically stable patients, with low rates of complications and need for re-interventions.
22.6 Kidney
After severe trauma, acute kidney injury (AKI) is a frequent and severe complication leading to increased morbidity and mortality. After renal trauma, additional risks have been identified due to direct parenchymal or vascular injuries [59].
Renal injuries are identified in approximately 1–5% of all polytraumatized patients, and they can be secondary to multiple mechanisms such as penetrating, blunt, and iatrogenic trauma. 80–90% of injuries are caused by blunt trauma, with the most common mechanism being traffic accidents.
After FAST assessment of the presence of solid abdominal organs injuries, diagnosis and extension of renal injuries is usually based on CECT imaging and graded according to the American Association for the Surgery of Trauma (AAST) organ injury scale (see Table 1 in Chap. 19). CECT is in fact considered the gold standard diagnostic method for the radiographic assessment of patients with renal trauma. With a short examination time, it can provide all the information related to the degree of renal injury, the eventual involvement of pyelo-caliceal system and the presence and type of vascular injuries; furthermore, CECT can also provide information about the renal functional status [60, 61].
Operative vs. non-operative management of traumatic renal injuries has been debated for many years. Traditionally, surgical exploration or nephrectomy represents the gold standard approach for high-grade renal injuries. However, on the basis of the advances in diagnostic imaging and the rise of minimally invasive techniques, NOM has become the standard of care. The majority of renal injuries are low-grade (grades 1–3 AAST organ injury scale) and can be conservatively treated. However, intervention may be needed in the presence of active arterial bleeding: surgery is usually reserved to haemodynamically unstable patients while for stable patients Renal Artery Embolization (RAE) should be considered [61].
Indications for RAE following trauma are debated. In cases of penetrating trauma, RAE is often a first-line alternative to surgery. For blunt trauma, the relative role of wait-and-see, RAE and surgical management may depend upon the grade of injury and patients’ haemodynamic conditions. Typically, RAE is indicated in the management of lower-grade renal injuries and penetrating injuries previously identified on CECT imaging [62]. However, Sarani and colleagues in a retrospective study compared angioembolization to surgery for the treatment of high-grade renal injuries (≥ grade III) concluding that RAE was a safe and effective technique to stop the bleeding and arrest haemorrhage [63].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree