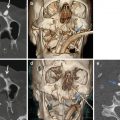
Fig. 14.1
Hepatic laceration. (a) Ultrasound shows a hypoechoic area within the right liver lobe (arrow); (b) CEUS: the laceration appears a nonvascular area (black arrow). The CE-MDCT, in arterial phase (c) and portal phase (d), confirms the presence of a liver laceration (arrows) involving segment IV; no active bleeding is appreciable in both CEUS and CE-MDCT imaging techniques.
Regarding traumatic liver injury, there is a good correspondence between CEUS and MDCT; false negatives with CEUS may be represented by minor injuries, without significant consequences for management and prognosis of patient [17].
Many studies have shown that it is possible to monitor the functionality of hepatic microcirculation, in those who suffered an ischemic damage with subsequent reperfusion of parenchyma, through the use of CEUS [18]. Thanks to this method, the dysfunction of liver microcirculation was evaluated and it was determined how the perfusion of contrast with microbubbles is negatively correlated with the severity of the injury [19].
Some authors suggest the use of CEUS following the execution of a standard ultrasound scan or ultrasound FAST, for those patients who have suffered a low-energy trauma and are hemodynamically stable. Therefore, CT scan would be reserved to high-energy trauma with suspected multiple organ injuries or in cases in which CEUS was not conclusive. CEUS showed a diagnostic accuracy almost comparable to the contrast-enhanced CT scan in the detection of abdominal parenchymal lesions associated with low-energy trauma, with a sensitivity and specificity close to 95% [20]. Another recognized role of CEUS is to be able to highlight blood extravasation and then the possibility of a subsequent therapeutic treatment in a short time.
Other applications are its use for pediatric patients and pregnant women, thanks to the absence of ionizing radiation and of harmful effects on fetus by ultrasound contrast agents. However, CEUS presents some limitations: operator-related method, high costs of contrast media, lack of panoramic view and difficulty to explore deep abdominal regions. Even if, as mentioned, CEUS proved to be more sensitive than baseline ultrasound in the detection of solid organ injury in blunt abdominal trauma, it cannot be deemed to replace CT scan in high-energy trauma. Also in case of isolated trauma, if CEUS detects a liver parenchyma injury, it must be followed by a MDCT examination.
CEUS represents a diagnostic supplement to CT and a radiation protective option in the follow-up of liver lesions, especially in young patients and children [21, 22].
14.4 Computed Tomography
Computed tomography (CT) is the diagnostic examination of choice for polytrauma hemodynamic stable patients. In fact, it represents a time-saving diagnostic technique avoiding the use of other imaging methods and so enabling the Trauma Team to start treatment ahead of time.
MDCT scan allows the panoramic study of the abdomen also in critical patients that may be partially or not collaborating because of their clinical conditions and their need of resuscitation procedures, and this rapid panoramic assessment might be essential to enable treatment onset in a short time [23]. The CT examination has high sensitivity (approximately 99%), specificity (about 96.8%), and diagnostic accuracy (about 97.6%) in the diagnosis of traumatic damage of the liver. It also allows the clinicians to monitor the lesions and highlight the possible occurrence of early and late complications over the time (seromas, bilomas, abscesses, necrosis, pseudo-aneurysm), either after conservative therapy or surgical/interventional treatment [24, 25]. MDCT can detect small amounts of free fluid, with sensitivity similar to that of US, but it is superior to US in pointing out injuries of the visceral organs. Moreover CT, compared with US, is less dependent on the operator, less limited by technical factors and is also reproducible [26].
Contrast-enhanced CT scan for polytrauma patients encloses the study of the head, neck, thorax, abdomen, and pelvis, sometimes even the lower and upper limbs are evaluated. It usually is a multiphase examination, with a basal phase followed by an arterial and portal venous phase. Arterial phase is fundamental for the study of vascular injuries, while venous phase is more useful to identify parenchymal liver injuries. It’s important to use an appropriate personalized contrast dose based on patient’s weight; the exam needs a high-flow intravenous injection (3–4 mL/s). In fact, the amount of contrast greatly improves the visibility of parenchymal lesions and thus the final quality of the examination. In addition to the axial scan, the post-processing with coronal and sagittal multiplanar reconstructions (MPRs) are routinely performed for the evaluation of abdominal structures while maximum-intensity projections (MIPs) and volume rendering (VR) reconstructions are required in case of vascular injuries.
In 1989, Mirvis et al. proposed a classification system of liver lesions based on the size of lesions and the number of involved segments visualized on CT [27]. This classification divides traumatic liver lesions in five degrees.



Grade I includes capsular avulsion, superficial laceration (<1 cm of thickness), sub-capsular hematoma (<1 cm of thickness), and periportal tracking.
Grade II includes laceration with thickness of 1–3 cm and central sub-capsular hematoma with a diameter of 1–3 cm.
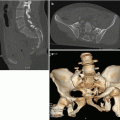
Grade III includes lacerations with thickness >3 cm and central sub-capsular hematoma with a diameter >3 cm (Fig. 14.2).
Grade IV includes sub-capsular hematoma with a diameter >10 cm and lobar parenchymal breaking or devascularization (Fig. 14.3, Fig. 14.4).
Grade V includes bi-lobar parenchymal breaking or devascularization [27].

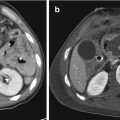
Fig. 14.2
Hepatic trauma, grade III: (a) Axial CT scan and (b) coronal reconstruction show multiple lacerations with thickness >3 cm

Fig. 14.3
(a) Axial CT scan and (b) coronal reconstruction show a grade IV parenchymal injury involving from 25 to 75% of a liver lobe (arrows)

Fig. 14.4
(a) Axial CT scan, (b) coronal, and (c) sagittal reconstruction show a IV grade parenchymal lesion, involving segment IV completely
Despite the usefulness of this classification for clinical research purposes, as it allows the description of the severity of injuries, it doesn’t seem to be helpful in clinical management because no correlation was reported between lesions grading and required treatment, as it was observed that even in high-grade lesions involving three or more segments, good clinical results can be achieved by conservative treatment [28]..
This CT-based classification system comes from the surgical grading drawn up by the American Association for the Surgery of Trauma (AAST) that divides hepatic trauma in six grades. (Table 14.1) [29] (Fig. 14.5).

Table 14.1
The American Association for the Surgery of Trauma (AAST) hepatic injury scale
Degree of injury | Description |
|---|---|
I | Hematoma: sub-capsular with liver surface’s involvement <10% |
Laceration: capsular lesion with depth <1 cm | |
II | Hematoma: sub-capsular, interest 10–50% of the surface |
Hematoma: intraparenchymal <10 cm in diameter | |
Laceration: capsular injury, depth 1–3 cm and length <10 cm | |
III | Hematoma: sub-capsular, surfaces’s involvement >50% or sub-capsular hematoma with signs of breaking and with active bleeding |
Hematoma: intraparenchymal >10 cm in diameter or with sings of breakage | |
Laceration: capsular lesion, depth >3 cm | |
IV | Hematoma: peritoneal rupture and hemorrhage in progress |
Laceration: parenchymal injury involving from 25 to 75% of a liver lobe or up to three segments of the same lobe | |
V | Laceration: parenchymal lesions involving over 75% of a liver lobe or more than three segments of the same lobe |
Venous injury: juxta-hepatic (intrahepatic lesion of inferior vena cava) or supra-hepatic veins | |
VI | Avulsion: of the vascular pedicle |
Laceration: parenchymal extended to both liver lobes |

Fig. 14.5
(a) Arterial phase axial CT scan shows a perihepatic fluid collection; there is no evidence of arterial bleeding. (b) Venous and (c) equilibrium phases well depict the active intraperitoneal bleeding
The surgical organ-injury scale of the AAST comprises some criteria that cannot be evaluated with CT and wide discrepancies have been seen between the CT injury grade and intraoperative findings, with CT usually underestimating injury gravity [30]. So several studies say that the CT-based injury-grading systems facilitate clinical research as they enable comparison of different series of patients, but their value for predicting the outcome of conservative treatment remains, however, unproven.
The traumatic hepatic injuries detected on CT may be summarized as follows:
Contusion: hypodense area with irregular margins resulting in interstitial blood suffusion. Rarely, contusion is an isolated post-traumatic liver injury, and it is usually associated with tearing or hematoma.
Laceration: it is the most common traumatic injury of the liver. It appears as a linear or branched image slightly hyperdense on direct examination, caused by the presence of blood and clots of recent deposition, and hypodense in contrast-enhanced scans [31].
Lacerations are often located in the proximity of an intrahepatic portal branch or a supra-hepatic vein; the most frequent lacerations are located parallel to the supra-hepatic veins or to the posterior division of the portal vein’s right branch.
They are classified according with their size into: superficial (extension <1 cm deep) (Fig. 14.6), medium (between 1 and 3 cm deep) (Fig. 14.7), and deep (extension >3 cm deep) (Fig. 14.8).




Fig. 14.6
(a) Axial CT scan and (b) coronal reconstruction show the superficial hepatic laceration (arrows): extension is <1 cm deep

Fig. 14.7
(a) Axial CT scan and (b) coronal reconstruction show a laceration of hepatic parenchyma between 1 and 3 cm deep (arrows)

Fig. 14.8
(a) Axial CT scan, (b) coronal, and (c) sagittal reconstruction show a deep hepatic laceration >3 cm deep (arrows in a) and (b). In (c), a huge hemoperitoneum is appreciable (asterisks)
Liver lacerations are categorized as deep when affecting the liver parenchyma adjacent to intrahepatic portal branches of first and second order, independently of the involvement of the peripheral parenchyma [29].
If laceration involves the whole surface of the liver, the outcome could be in liver fracture with avulsion of a fragment devoid of vascularization. Depending on the location of the lacerations, they may be associated with other post-traumatic alterations: hemoperitoneum in capsular tears of anterior liver surface, retroperitoneal hematomas adjacent to the inferior vena cava and to the right adrenal gland (“halo sign”) in tearing interesting liver’s nude area, damage of the biliary tract (hemobilia or bilomas) in tears near the hepatic hilum.
Liver lacerations are particularly serious when affecting the confluence between the supra-hepatic veins or intrahepatic vena cava, in this case resulting in massive venous bleeding.
Hepatic infarction: in this case, we see an ischemic area in the liver with irregular margins. It is hypodense in basal phase and doesn’t show enhancement during the contrast phase (Fig. 14.9). It’s generally caused by thrombotic obstruction or post-traumatic avulsion of one of the main hepatic vessels; the greater is the caliber of the vessel involved, the greater will be the ischemic area.


Fig. 14.9
(a, b) Axial CT scan in arterial (a), venous phase (b) and (c) coronal reconstruction show the hepatic infarction as a hypodense triangular-shaped area (arrows). Hepatic infarction is an ischemic area with irregular margins, which doesn’t show enhancement during contrast-enhanced phase
Hepatic infarction may extend to involve the whole liver in the event of avulsion of vascular pedicle. Disruption of the main vessel can also be associated with massive intraparenchymal or peritoneal hemorrhage, which requires immediate surgical treatment. The rupture of the inferior vena cava or supra-hepatic veins, which cause intraparenchymal and/or extraperitoneal massive bleeding, is a rare and potentially lethal complication [28].
Periportal tracking: this sign is not specific for trauma lesion; it is represented as a hypo-density of periportal fat tissue (Fig. 14.10). It can be determined by:

- 1.
Post-traumatic blood suffusion of periportal interstitium
- 2.
Distension of the periportals lymphatic vessels and lymphedema, which can happen after a rapid increase in central venous pressure (rapid fluid infusion to stabilize the patient, hypertensive pneumothorax, cardiac tamponade, hematoma compressing supra-hepatic veins)

Fig. 14.10
(a, b) Axial CT scans and (c) coronal reconstruction show the periportal tracking characterized by hypodensities of periportal fat tissue (arrows)
Sub-capsular hematoma: it is a blood collection with biconvex margins between the capsule and liver parenchyma. It appears as hyperdense at direct CT scan and hypodense during contrast-enhanced phase. It is almost always associated with liver lacerations on the surface and generally it is localized along the antero-lateral surface of the right lobe. In contrast to the free fluid in peritoneal cavity, the sub-capsular hematoma determines compression on the adjacent liver parenchyma. This type of hematoma is rare in blunt abdominal trauma, but represents a frequent complication of invasive procedures such as percutaneous liver biopsy.
Intraparenchymal hematoma: is a collection of blood which is developed in the context of a liver laceration. It appears as a round hyperdense area on direct CT scan (45–70 U.H, typical density of coagulated blood) with no enhancement after contrast media administration (Fig. 14.11). If air bubbles appear in the context of collecting blood, you could think of a bacterial superinfection by anaerobic bacteria or necrotic events. These elements are a negative prognostic factor, furthermore yet allowing a conservative treatment [34].


Fig. 14.11
(a) CT scan without contrast media administration, (b, c) CE-MDCT scan in arterial (b) and in venous phase (c) depict a parenchymal hematoma. The hematoma appears as a round hyperdense area in (a) (arrow), and hypodense in the post-contrast phases (b, c) (arrows)
14.4.1 Vascular Contained Lesions: Arterial Pseudo-Aneurysm and Arteriovenous Fistula
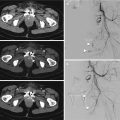
Arterial pseudo-aneurysm: it is also known as a false aneurysm, and it is represented by a collection of blood between the two outer layers of an artery, muscularis and adventitia (Fig. 14.12). It’s more frequent in open abdominal trauma. In blunt abdominal trauma, it can occur as a result of a bile extravasation. Usually, it can be found in proximity of a hepatic laceration that determines intimal lesion of the vessel.


Fig. 14.12
Hepatic trauma treated by surgical packing. (a) Axial CT scan and (b) coronal reconstruction show a pseudo-aneurysm: it appears as a focal lesion with high attenuation values in the arterial phase (arrows)
On CT, it appears as a focal lesion with high attenuation values and featuring similar to normal arteries on contrast-enhanced scans. The morphology of the pseudo-aneurysm does not change during the phases of the exam, and this is important for differentiate it from a pooling. It may not be present at the first examination but it can appear in the following exams. In case of rupture, it can cause massive bleeding, fistula with biliary tract, intra-duodenal rupture, and consequent bleeding of the upper gastro-intestinal tract.
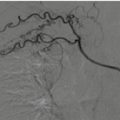
The demonstration of its presence is associated with the need for timely selective embolization of the affected vessel using angiography.
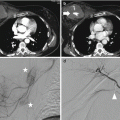
Arteriovenous fistula is a rare event, usually generated by a penetrating hepatic trauma that causes a communication between a main hepatic artery and portal vein or between the hepatic artery and a supra-hepatic vein [35] (Fig. 14.13). In contrast-enhanced CT scans, fistula remains enhanced in both phases, arterial and venous.


Fig. 14.13
Arteriovenous fistula in the arterial (a), venous (b), and equilibrium (c) phases; it is a communication between the main hepatic artery and a portal vein (arrows)
The differentiation between arteriovenous fistula and pseudo-aneurysm is easy to perform with angiography that is also useful in therapeutic approach.
Active bleeding: Pooling of contrast material on CT indicates free extravasation of blood as a result of active bleeding (Fig. 14.14). The presence of active bleeding is an important risk factor for the patient since it can be the cause of hemodynamic instability and determine the need for a timely surgical intervention. The contrast-enhanced CT scan can detect the presence, location, and extent of an active bleeding [36].


Fig. 14.14
Active bleeding: the extravasation of contrast in the arterial phase (a) becomes stronger in the venous (b), and the equilibrium (c), phases. (d) Coronal CT reconstruction confirms the active bleeding (arrow)
Active arterial bleeding has become evident due to the extravasation of contrast medium in the arterial phase in the context of a hematoma, liver laceration, or peritoneal collection. During venous and late phases, the area of active bleeding spreads out. The attenuation values of an active bleeding are >90 U.H; therefore, higher than those of the coagulated blood and the liver parenchyma. To date, the recognition of an active arterial bleeding is an absolute indication to the embolic treatment through angiography.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree