- •
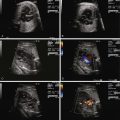
Identify the presence, position, and location of the ductus arteriosus as originating from the confluence of the branch pulmonary arteries off of the main pulmonary artery and inserting into the descending aorta distal to the left subclavian.
- •
Identify the shape and contour of the ductus arteriosus and the ductal arch.
- •
Perform Doppler interrogation of the ductus arteriosus and determine the direction of flow (normally antegrade from pulmonary artery to aorta).
- •
Assess the ductus arteriosus for evidence of constriction via two-dimensional imaging, color flow imaging, and Doppler interrogation.
- •
If there is evidence for constriction of the ductus arteriosus, assess the right ventricle for any hypertrophy or dysfunction and assess the tricuspid valve for any regurgitation and right ventricular pressure estimate.
- •
When there is structural heart disease, take particular note of the direction of flow across the ductus arteriosus within the context of the presence or absence of pulmonic or aortic obstruction.
The ductus arteriosus (DA) is a structure that connects the pulmonary artery to the descending aorta and is the conduit for blood exiting the right ventricle (RV) to the aorta. The DA has a very characteristic shape and contour that directs flow between the RV and the aorta. Sixty percent of the total fetal cardiac output exits the RV with the majority diverted down the DA. A relatively small amount of blood flow enters the branch pulmonary arteries to perfuse the developing pulmonary vasculature and nourish the immature lung parenchyma. The amount of blood entering the lungs increases with gestation, with approaching up to 25% of the cardiac output perfusing the branch pulmonary arteries near term.
Structural abnormalities of the DA exist and can have a significant impact on fetal blood flow patterns. Impediment to flow across the DA not only affects downstream blood delivery to the descending aorta but may also alter flow into the branch pulmonary arteries or create alterations in flow patterns upstream such as at the atrial level. These changes in flow can influence cardiovascular development leading to various pathological conditions.
Structural abnormalities can include (1) constriction or premature closure of the DA, (2) agenesis of the DA, or (3) aneurysm of the DA.
Constriction or Premature Closure of the Ductus Arteriosus
Anatomy, Development, and Prenatal Physiology
Constriction or complete premature closure of the DA is an unusual phenomenon with potentially devastating consequences. Premature closure of the DA results in an undue afterload on the RV because the pathway to the descending aorta is obliterated and pulmonary vascular resistance is naturally very high. It is associated with right ventricular hypertrophy and tricuspid regurgitation. RV pressure can be increased to levels higher than normal. Right ventricular hypertrophy alters ventricular compliance, which induces more right-to-left shunting at the atrial level, with reduction in volume delivered to the RV and pulmonary arteries. As a result, blood flow patterns to the lungs are markedly affected with increased pulmonary artery pressure leading to the potential for development of pulmonary vascular disease. An outline for possible outcomes after premature closure of the ductus arteriosus has been proposed ( Figure 36-1 ).

Constriction of the DA is likely a more common, fleeting event than is complete premature closure, and hence, it is unknown how often it takes place. Constriction or temporary “spasm” of the DA, with relaxation and return to normal caliber, is possible.
Constriction or premature closure of the DA is due to known extrinsic agents that cause ductal constriction, or it is “idiopathic,” which essentially means it is likely also caused by some extrinsic agent; however, we cannot identify what that agent may be. Nonsteroidal anti-inflammatory drugs such as indomethacin, as well as salicylates, are well-known constrictors of the fetal DA. In our experience, we have also found that maternal intake of sympathomimetics, such as those contained in over-the-counter cold remedies, may predispose to DA constriction. Colleagues in South America described fetal constriction of the DA with excessive intake of “maté” beverage, a popular, hot drink derived from the steeping of yerba maté leaves. Furthermore, we once identified serious constriction of the DA in a native African woman. Upon questioning, she revealed the use of a cream derived from local African plants, which was being applied to her gravid abdomen. The purpose of the cream, extremely popular in her native Kenya, is to improve the abdominal skin condition and prevent the development of unsightly stretch marks after birth. Of course, the precise herbal contents of this cream are unknown. These anecdotes convey the point that constriction of the DA can be influenced by a myriad of agents, most of which are unknown to the medical practitioner.
The consequences of prenatal ductal constriction are primarily changes in the morphology of the RV with myocardial thickening and development of tricuspid regurgitation. Systolic dysfunction and right-sided heart failure ensues, possibly leading to fetal hydrops. Long-standing constriction or complete premature closure can lead to right ventricular hypertrophy, right ventricular hypoplasia with pulmonary valve stenosis, or even atresia, the latter being related to chronic diversion of blood away from the right-sided structures. Persistent pulmonary hypertension in the newborn due to abnormal pulmonary vascular development is the most feared consequence and can be difficult to manage.
Prenatal Diagnosis
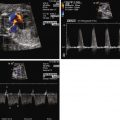
Constriction of the DA is oftentimes first suspected when there is fetal echocardiographic evidence for ventricular size discrepancy, with a dilated and hypertrophied RV in comparison with the left ventricle. Greater than mild tricuspid regurgitation is common, but not necessarily present in all cases. Inspection in the region of the DA will reveal a narrowed structure. Color Doppler flow will demonstrate a high-velocity jet emanating from the orifice of the constricted DA directed into the descending aorta. Doppler echocardiography will reveal an increased systolic and diastolic velocity. The pulsatility index (PI) will be decreased. The PI for a normal DA is typically 2.0 to 2.5, whereas in the constricted DA, it is typically less than 1.8. In a reported series of 22 fetuses with constriction of the DA due to indomethacin treatment, the average DA PI was 1.25.
Two-dimensional measures of the DA have been described; however, such measurements can be difficult to obtain. The width diameter of the DA increases with gestation ( Figure 36-2 ).

Management and Outcomes
Identification of a constricted DA, or premature closure, warrants close serial scrutiny with fetal echocardiography for any deleterious consequences. Right ventricular dysfunction with severe tricuspid regurgitation and development of hydrops is possible. Serial assessment looking for fusion of the normal dual peak inflow pattern across the tricuspid valve, increased reversal of ductus venosus flow with atrial contraction, and umbilical venous pulsations is required, because these may herald the onset of heart failure and hydrops. Careful history taking may reveal an inciting agent, which should be eliminated. In cases of impending hydrops or severe right ventricular dysfunction, early delivery should be considered. Delivery will result in opening of the pulmonary vasculature and relief of right ventricular afterload. The neonate can then be further treated with pulmonary vasodilating agents such as nitric oxide. Postnatal diagnosis is often made of premature closure of the DA when there is marked cyanosis, a small right ventricular cavity with thickened ventricular myocardium, and the absence of visualization of a patent DA within the first 24 hours of life.
Outcome depends upon the degree, duration, and developmental stage at which DA constriction has taken place. In mild cases, elimination of the inciting agent can result in complete normalization without any further consequences. In severe cases, when pulmonary vascular disease is present, death can occur with placental separation despite aggressive intervention. An intriguing additional notion has recently been raised. Based on the theory of “fetal origins” of adult-onset cardiovascular disease (Barker’s hypothesis), it is plausible that “primary” pulmonary hypertension, a disease whose origins are poorly understood, may be founded in the altered fetal flow patterns of ductal constriction leading to a developmental predisposition to this disorder.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree