Early clot lysis may prevent or significantly decrease the incidence and severity of postthrombotic syndrome after acute lower extremity deep vein thrombosis (DVT). Several smaller studies comparing systemic thrombolytic therapy for acute DVT with anticoagulation showed that significant or complete clot lyses can be achieved by giving systemic thrombolysis, but with an unacceptably high rate of bleeding complications.1,2 Catheter-directed thrombolysis for DVT has proven to be a safe and effective method to remove acute iliofemoral DVT.3,4 Mechanical thrombectomy has emerged as an alternative or adjunct to pharmacologic thrombolysis.5 Population-based studies indicate that the annual incidence of DVT is between 122 and 160 per 100,000.6 It is thought that approximately 10% of patients with lower extremity DVT will have iliac vein involvement; these patients have an especially high risk of developing severe postthrombotic syndrome, and this may vary significantly. A recently published article from a large tertiary care facility found that 24% of patients diagnosed with DVT did have involvement of the iliac veins.7 The long-term sequelae of lower extremity DVT are very significant, as has been documented by many authors. Up to two thirds of patients with acute DVT treated according to accepted algorithms will develop postthrombotic syndrome. Patients with iliac vein thrombosis have a much worse prognosis.8–10 There are no well-established indications for thrombolytic therapy for iliofemoral DVT. The challenge lies in the lack of conclusive randomized studies that document the safety and efficacy of thrombolysis compared to conventional anticoagulation. The decision to offer thrombolytic treatment to a patient with DVT has to be weighed carefully, considering the anticipated clinical outcome of the procedure and potential risks. The decision therefore becomes individualized, taking into account age and comorbid factors such as cancer or bleeding diatheses. Based on several small and large case studies, it is believed that certain indications can be supported on an individual basis.3,4,11 The Society of Interventional Radiology (SIR) and the Cardiovascular and Interventional Radiology Society of Europe published quality improvement guidelines in 2006 that suggest certain indications. These are listed in Table e101-1 TABLE e101-1 Indications for Catheter-Directed Venous Thrombolysis *Bleeding risk for thrombolysis. Modified from Vedantham S, Thorpe PE, Cardella JF, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol 2006;17:435–48. The largest groups of patients currently considered for thrombolytic therapy are patients with acute iliofemoral DVT. These patients are at high risk of developing postthrombotic syndrome and recurrent DVT and are frequently very symptomatic at first presentation. The potential of changing the long-term outcomes for these patients is therefore quite significant. Catheter-directed thrombolysis may be an acceptable alternative treatment and possibly superior to conventional anticoagulation in patients with a long life expectancy and a low risk for bleeding.14 A larger group of patients in whom the indications are less studied are patients with acute femoropopliteal DVT. Earlier studies using systemic thrombolysis for femoropopliteal thrombosis did indicate that there was a potential benefit from thrombolysis.1,2 It has to be borne in mind that many patients with femoropopliteal DVT will become free of symptoms if treated conventionally with anticoagulation and compression garments. Based on this, the threshold for catheter-directed thrombolysis should be higher for femoropopliteal DVT than for iliofemoral DVT. Catheter-directed thrombolysis should be reserved for patients with severe symptoms from femoropopliteal DVT and those who experience progression of thrombosis on anticoagulation.14 A limited number of practitioners use thrombolytic therapy in conjunction with iliac vein recanalization in patients with chronic iliac vein thrombosis or subacute thrombosis. This practice is discouraged and probably has very limited benefits in this group of patients.2,14 Phlegmasia cerulea dolens, an acute fulminating form of DVT characterized by reactive arterial spasm, pronounced edema, severe cyanosis, petechiae, and purpura, carries a high risk of amputation and significant morbidity and mortality.15–17 Many patients have significant comorbid factors such that any correctible management—surgical, endovascular, or medical—carries high risk. Several case reports of catheter-directed thrombolysis for phlegmasia cerulea dolens have been published17,18 describing good short-term clinical improvement. Catheter-directed thrombolysis is found to be justified for most patients with this condition if they do not have a high risk of bleeding. When bleeding risk is believed to be prohibitory for thrombolysis, surgical thrombectomy should be considered. This is regarded as an emergent procedure.14 Acute thrombosis of the inferior vena cava is often very symptomatic, leading to bilateral lower extremity leg and genital congestion. If the thrombosis extends above the renal and hepatic veins, renal failure and Budd-Chiari syndrome may result. Filter placement may not be possible to prevent pulmonary emboli. For these reasons, catheter-directed thrombolysis may be justified to prevent or relieve the sequelae mentioned earlier.14 Both the American College of Chest Physicians19 and the American Heart Association20 have now included catheter-directed thrombolysis as an option for treatment of selected patients with lower extremity deep vein thrombosis. Contraindications to venous thrombolysis and mechanical thrombectomy are practically the same as for any other thrombolytic therapy and are listed in Table e101-2. TABLE e101-2 Contraindications to Catheter-Directed Venous Thrombolysis Modified from Vedantham S, Thorpe PE, Cardella JF, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol 2006; 17:435–48. The equipment needed is the same as that used for arterial thrombolysis. Additional equipment is needed for mechanical thrombectomy. The main items needed are listed in Table e101-3. TABLE e101-3 The large number of mechanical thrombectomy devices available may be an indicator of their shortcomings thus far. They can be divided into so-called wall contact devices and non–wall contact devices (Table e101-4 TABLE e101-4 Mechanical Thrombectomy Devices Modified from White RH, Zhou H, Kim J, Romano PS. A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med 2000;160:2033–41. The anatomy of the lower extremity, iliac veins, and inferior vena cava is familiar to all interventionists, but the nomenclature of some of the lower extremity veins has recently changed.22 For example, the superficial femoral vein is now called the femoral vein.23 The following is a short practical review with correlation to the approach for venous thrombolysis. It is important to recognize that the anatomy of the lower leg veins varies. The three leg veins are paired and travel parallel to the main leg arteries and bear the same names: the posterior tibial vein, anterior tibial vein, and fibular or peroneal vein. The posterior tibial vein pair traverses behind the medial malleoli with the posterior tibial artery. The anatomy here is important because access can be gained into the posterior tibial vein at this level. This access can be used for placement of infusion catheters through which thrombolytic agents can be infused into thrombosed popliteal vein. The deep leg veins communicate with the superficial veins via perforating veins. The perforating veins have been used for placement of infusion catheters into thrombosed deep veins.24 The leg veins will then merge and form the popliteal vein. The anatomy in the popliteal fossa is quite variable.25 The vein is often partially or entirely duplicated. The femoral vein is quite consistent even though it can be duplicated (21.2%) or even have multiple channels (13.8%).26 Usually only one infusion catheter is placed, so in the case of a split femoral vein, one has to select one channel to infuse into. The femoral vein will then merge with the profunda femoris vein, forming the common femoral vein. One major branch drains into it, the great saphenous vein (formerly the greater saphenous vein). The transition to the external iliac vein is at the inguinal ligament and the deep circumflex iliac vein, which is a tributary and anatomic landmark. As the internal iliac vein joins the external iliac vein, the common iliac vein is formed. The right and left common iliac veins will subsequently form the inferior vena cava, which will ascend to the right and anterior of the spine to the right atrium. A variant that is commonly seen with iliac vein thrombosis is compression of the left common iliac vein by the right common iliac artery crossing it anteriorly. Commonly, a stenosis is present at this point in the left common iliac vein, and the vein is often septate or webbed, which causes partial or complete obstruction with variable collateral formation. It has been estimated that between 14% and 30% of these patients have May-Thurner syndrome.27 The right internal jugular vein was the access vein of choice during the infancy of the procedure. As interventionists became more experienced, the trend swayed subsequently to popliteal vein access (or small saphenous vein) and the posterior tibial vein.4,11
Acute Lower Extremity Deep Venous Thrombosis
Indications
![]() .
.
Indications
Purpose
Bleeding Risk*
Life Expectancy
Acute iliofemoral deep venous thrombosis
Prevent postthrombotic syndrome
Low
Long
Acute femoropopliteal deep venous thrombosis
Prevent postthrombotic syndrome
Low
Long
Subacute and chronic iliofemoral deep venous thrombosis
Prevent postthrombotic syndrome
Low
Long
Phlegmasia cerulea dolens
Limb salvage
Low-moderate
Any
Acute or subacute inferior vena cava thrombosis
Preserve organs, symptom relief
Low-moderate
Any

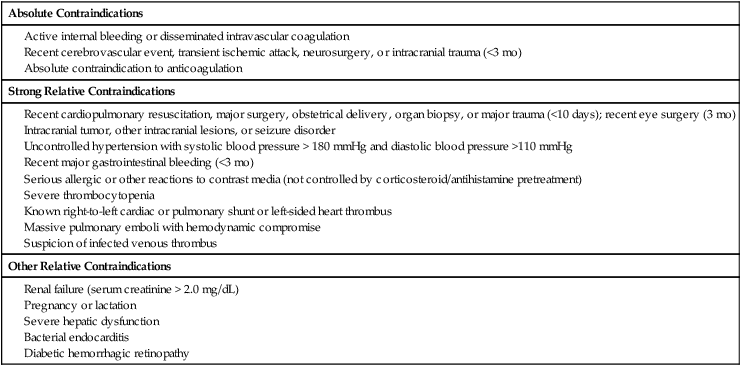
Contraindications
![]()
Equipment
![]()
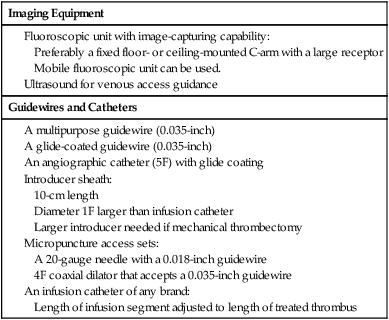
Imaging Equipment
Guidewires and Catheters
![]() ). Many of the devices have undergone preclinical and clinical evaluation for DVT and have been found to be effective, with very little trauma to the valvular elements, which is one of the main concerns. Concomitant pulmonary emboli have been one of the concerns with mechanical thrombectomy, and placement of an inferior vena caval filter has been recommended with some devices.21
). Many of the devices have undergone preclinical and clinical evaluation for DVT and have been found to be effective, with very little trauma to the valvular elements, which is one of the main concerns. Concomitant pulmonary emboli have been one of the concerns with mechanical thrombectomy, and placement of an inferior vena caval filter has been recommended with some devices.21
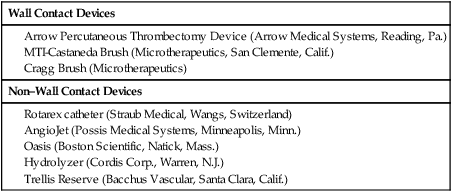
Wall Contact Devices
Non–Wall Contact Devices
Technique
Anatomy and Approaches
Anatomic Considerations
Access
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Acute Lower Extremity Deep Venous Thrombosis