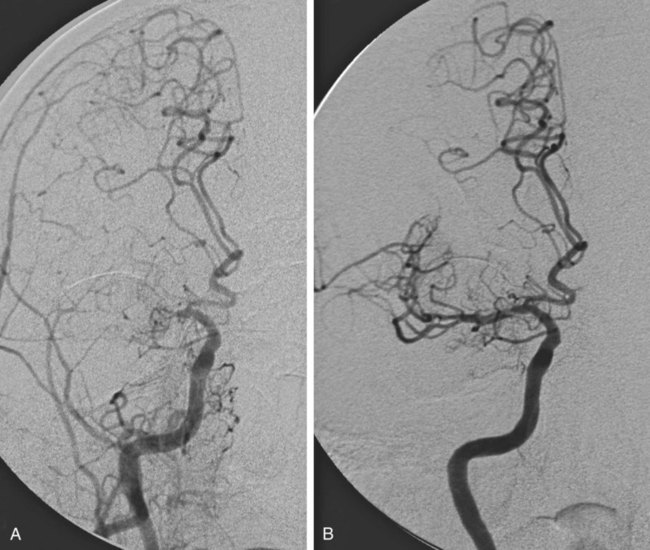
Stroke is the leading cause of disability and the third leading cause of death in the United States, with approximately 795,000 patients a year experiencing a stroke, at an annual cost of $40.9 billion.1 Almost 90% of strokes are ischemic and arise from arterial thrombotic or thromboembolic occlusion. Loss of cerebral blood flow results in cerebral ischemia, which cascades to infarction if brain perfusion is not rapidly restored. The goal of acute ischemic stroke (AIS) intervention is to minimize cerebral damage by restoring blood flow to ischemic brain tissue as efficiently and safely as possible. In 1995, results from the National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group2 demonstrated that in carefully selected ischemic stroke patients, significant clinical benefit at 3 months is observed after intravenous (IV) administration of tissue plasminogen activator (tPA) performed within 3 hours of AIS onset. More recent studies have shown that the window for treatment could be extended to 4.5 hours in certain patients.3,4 Despite the benefits of IV tPA, which resulted in U.S. Food and Drug Administration (FDA) approval for the treatment of AIS, the utility of the drug for the vast majority of AIS patients is limited in actual practice. Because of the limited time window and other stringent exclusion criteria (Table 92-1), only 2% to 6% of stroke patients are administered IV tPA.5,6 When treated with IV tPA alone, patients with large-vessel ischemic stroke, which carries the highest morbidity and mortality rate, still have overall poor clinical outcomes, most likely because IV tPA fails to recanalize up to 70% of large artery occlusions.7 Endovascular stroke intervention is aimed at treating these large-vessel strokes and expanding the proportion of the AIS population eligible for acute treatment. TABLE 92-1 Exclusion Criteria for Intravenous Administration of Tissue Plasminogen Activator (tPA) BP, Blood pressure; CT, computed tomography; NIHSS, National Institutes of Health Stroke Scale. Careful patient selection for endovascular acute stroke treatment is paramount to successful clinical outcome. Ideally, at the time of the intervention, the patient should have minimal cerebral infarction, with a large ischemic penumbra that will revitalize once cerebral blood flow is restored. This should ensure maximal clinical benefit and help minimize the principal risk of endovascular AIS therapy, namely intracerebral hemorrhage (ICH). Table 92-2 summarizes the indications, which represent common knowledge within the neuro-interventional community at the time of this writing. It is important to note that mechanical embolectomy using the MERCI and Penumbra devices is not FDA-approved for the specific clinical indication of AIS. Use of these devices is based on single-arm trials sponsored by the respective companies (Concentric Medical and Penumbra), designed to gain 510K clearance from the FDA for the purpose of vessel recanalization and/or thrombus removal. As for intraarterial (IA) thrombolysis, although such treatment is based on the positive results of the randomized PROACT II trial (Prolyse in Acute Cerebral Thromboembolism),8 these results were not sufficient to achieve FDA approval. TABLE 92-2 Indications for Endovascular Acute Stroke Therapy NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator. *Internal carotid artery (including bifurcation, or “T” occlusions), middle cerebral artery (first and second divisions), vertebral artery, basilar artery, or proximal posterior cerebral artery. †≤12 hours for basilar artery occlusion, although efficacy not proved. Patients eligible for interventional stroke therapy include those arriving within 3 hours of stroke onset who have systemic contraindications to IV tPA treatment, as listed in Table 92-1. Endovascular stroke therapy is also indicated for patients arriving after 3 to 8 hours from stroke onset, because mechanical embolectomy and IA thrombolysis theoretically carry a lower risk of ICH than IV tPA after recanalization if performed under the same conditions. The likelihood of postrevascularization ICH depends on both temporal and tissue factors, as well as the extent of the induced coagulopathy. Large tissue infarctions, especially involving the basal ganglia, and prolonged duration of ischemia both elevate the risk of reperfusion hemorrhage. The duration and extent of ischemia determine the degree of neuronal death and cerebral tissue infarction, which progress in a time-dependent fashion.9 Although these factors are present regardless of treatment, it is the minimalization and even omission of a thrombolytic drug that enables the prolonged time window associated with IA therapy. The upper limit of this time window, however, is not certain. Data from the PROACT II trial, a randomized controlled trial of IA prourokinase, suggest that the upper limit for recanalization should be 8 hours after symptom onset. In the special case of basilar artery occlusion, these time-to-treat guidelines may not apply, and some centers will treat aggressively up to 24 hours after stroke onset, given the dismal 80% to 90% mortality rate associated with untreated acute basilar artery occlusion and the numerous series showing some positive outcomes after late endovascular recanalization.10 A key concept to consider when treating AIS, besides the time from symptom onset, is the amount of viable brain tissue at risk for infarction (penumbra) and the amount of core infarction present. Most would agree that patients with minimal or no core infarction and a large amount of penumbra are good candidates for endovascular treatment, regardless of the time from symptom onset, whereas patients with a large amount of core infarction (>100 mL) are not considered to be good candidates for endovascular treatment, owing to the higher risk of hemorrhage. Higher risk of hemorrhage may make even patients with greater than 70 mL of core infarction unsuitable candidates for endovascular treatment. Interventional stroke treatment is limited to patients with significant neurologic deficits, namely patients with National Institutes of Health Stroke Scale (NIHSS) scores greater than 4, except for patients with isolated aphasia or hemianopsia. In addition, patients with fluctuating symptoms and evidence of a large-vessel occlusion, even with little deficit at the time of treatment, may qualify for endovascular therapy. These patients typically have sufficient collateral circulation to maintain adequate cerebral blood flow to minimize neurologic deficits, but this compensation is nearly invariably temporary. IA stroke treatment is also indicated for patients who fail to recanalize after IV tPA administration. Finally, endovascular revascularization requires the angiographically demonstrable arterial occlusion of an accessible artery. Appropriate target vessels include the internal carotid artery (ICA), the first (M1) and second (M2) division of the middle cerebral artery (MCA) (Fig. 92-1), the vertebral artery, the basilar artery, and the first (P1) segment of the posterior cerebral artery. Endovascular stroke therapy is contraindicated in patients with ICH, large established infarction (>one third MCA territory computed tomography [CT] hypodensity or magnetic resonance imaging [MRI] diffusion-weighted abnormality), or completed infarction without salvageable territory. CT perfusion, MRI diffusion/perfusion-weighted (DWI-PWI) mismatch, and MRI diffusion-weighted/clinical mismatch are all useful in determining the extent of the latter and aid in patient selection, especially when contemplating late recanalization (>3 hours). A DWI-PWI mismatch may be found in up to 70% of patients imaged within 6 hours from stroke onset,11 and it is hypothesized that these patients may derive the most clinical benefit from endovascular intervention. Some relative contraindications to IA therapy include rapidly improving neurologic examination, NIHSS over 30, and a history of anaphylaxis to thrombolytic drugs or iodinated contrast material. Digital biplane angiography with road-mapping capability is optimal for the safe and efficient performance of endovascular acute stroke intervention. General endotracheal anesthesia is required for most procedures, although monitored conscious sedation is at times adequate for cooperative patients. Our experience is that patients with left hemisphere strokes are paradoxically more cooperative than right hemisphere stroke patients. The patient should have an arterial line placed for blood pressure management, which may become crucial after recanalization to prevent reperfusion hemorrhage. In the time before vessel recanalization, it is critical to maintain adequate perfusion pressure by elevating the patient’s blood pressure to significantly above the patient’s baseline values, particularly during administration of general anesthesia. A recent study comparing outcomes of AIS patients12 showed that patients placed under general anesthesia during endovascular treatment for anterior circulation had higher rates of poor neurologic outcome and mortality, a difference that could potentially be due to lack of adequate blood pressure support during general anesthesia. Common femoral artery access requires a minimum of a 5F sheath for IA thrombolysis and up to a 9F sheath for the Merci Retrieval System. A 5F diagnostic catheter is used for cerebral angiography to demonstrate and characterize the arterial occlusion. The catheter and sheath are maintained under continuous heparinized saline flush (5000 units heparin per liter normal saline), and nonionic iodinated contrast (Omnipaque [GE Healthcare, Piscataway, N.J.]) is used for angiography. Once an intervention is deemed appropriate, minimal systemic anticoagulation is initiated with 2000 to 3000 units of IV heparin. A guide catheter is required for device support during thrombolysis and embolectomy. A microcatheter and microwire facilitate navigation past the occlusion, and the microcatheter provides a conduit for IA thrombolytic infusion. More specific details about the basic equipment required for IA treatment are summarized in Table 92-3. TABLE 92-3 Basic Equipment for Acute Stroke Management *Long 90-cm sheath may be used for support instead of a guide catheter for mechanical embolectomy, intracranial angioplasty, and stenting. The most commonly used drugs for IA thrombolysis include plasminogen activators such as urokinase, alteplase, reteplase, and prourokinase. Other newer thrombolytics that are not plasminogen dependent include V10153 (Vernalis, Winnersh, U.K.), a recombinant variant of human plasminogen, Microplasmin (ThromboGenics, Heverlee, Belgium), a truncated form of plasmin, Alfimeprase (Nuvelo, San Carlos, Calif.), a truncated form of fibrolase that degrades fibrin directly, and Ancrod, a purified fraction of the venom of the Malayan pit viper, which acts by directly inactivating fibrinogen. Given the prothrombotic activity of fibrinolytics, there is some rationale for adjunctive anticoagulant therapy when administering fibrinolytics in the setting of AIS. Investigations are ongoing involving combinations of fibrinolytics, anticoagulants, and platelet disaggregants for IV, IA, and combined IV/IA AIS therapy, although there is currently no clear evidence to recommend one combination over another. Periprocedural anticoagulation with IV heparin may allow for augmentation of thrombolysis and prevention of arterial reocclusion at the expense of potentially higher rates of ICH. The use of glycoprotein IIb/IIIa antagonists in ischemic stroke remains investigational; only limited data are available, mainly involving IV administration of a glycoprotein IIb/IIIa antagonist in conjunction with IV thrombolysis. A randomized double blinded placebo-controlled study in which 54 ischemic stroke patients were randomly selected to receive IV abciximab did not report a single ICH complication in the group receiving abciximab.13 The CLEAR trial evaluated the combination of low-dose IV rtPA and eptifibatide, a glycoprotein IIb/IIIa antagonist, in patients with NIHSS scores of less than 5 presenting within 3 hours of stroke onset. The study showed a nonsignificant trend toward increased efficacy with the standard-dose rtPA treatment arm, and there was a non–statistically significant increased incidence on ICH in the eptifibatide group.14 A small study on the IA administration of glycoprotein IIb/IIIa antagonists as adjunctive therapy in patients with large-vessel occlusion and AIS also suggests that the added risk of ICH is low.15 However, the AbESTT II trial, a phase III multicenter randomized double-blind placebo-controlled study evaluating the safety and efficacy of IV abciximab in AIS treated within 6 hours after stroke onset or within 3 hours of awakening with stroke symptoms, was stopped early because of high rates of symptomatic ICH in the abciximab-treated patients (5.5% vs. 0.5%, P = .002).16 Mechanical embolectomy involves the use of devices for mechanical clot extraction or fragmentation. The only two devices currently approved by the FDA for revascularization are the Merci Retriever (Concentric Medical Inc., Mountain View Calif.) and the Penumbra Reperfusion System (Penumbra Inc., San Leandro, Calif.). A myriad of other devices designed for this same purpose have recently become available in this regard, but their usage is off-label and/or investigational. Among these newer devices, the Solitaire and Trevo devices seem to be the most promising. The mechanisms employed by these devices include thrombectomy, thromboaspiration, mechanical disruption, angioplasty, stenting, laser techniques, ultrasound-based methods, flow augmentation, and transvenous retrograde reperfusion. A summary of the devices currently available for IA treatment of AIS is listed in Table 92-4. TABLE 92-4 Current Mechanical Embolectomy Devices Approved or Under Investigation
Acute Stroke Management
Indications

Contraindications
Equipment
Device
Example
Company
Femoral sheath
5F-9F, 11-90 cm*
Cordis Corp., Miami Lakes, Fla.
Diagnostic catheter
TempoVert
Cordis
Guide catheter*
6F Envoy
Cordis
Guider Softip XF guide catheter
Boston Scientific, Natick, Mass.
Shuttle Introducer
Cook Inc., Bloomington, Ind.
Balloon Guide Catheter
Concentric Medical Inc., Mountain View, Calif.
Intermediate catheter
DAC
Concentric Medical
Microcatheter
Excelsior SL10
Boston Scientific
Excelsior 1018
Boston Scientific
Prowler Plus
Cordis
Microguidewire
Transend-14
Boston Scientific
Synchro-14
Boston Scientific
Thrombolytic
tPA
Genentech Inc., San Francisco, Calif.
Abciximab (ReoPro)
Eli Lilly, Indianapolis, Ind.
Device
Company
Merci Retriever*
Concentric Medical, Mountain View, Calif.
Penumbra System*
Penumbra Inc., San Leandro, Calif.
Solitaire
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access