and Arumugam Rajesh2
(1)
Department of Radiology, Harvard Medical School Massachusetts General Hospital, Boston, Massachusetts, USA
(2)
Department of Radiology, University Hospitals of Leicester NHS Tr Leicester General Hospital, Leicester, UK
Case 1.1
Brief Case Summary:
35 year old male with abdominal pain
Imaging Findings
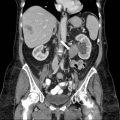
An axial non-contrast CT scan demonstrates a hypodense nodule in the right adrenal gland with attenuation value of 3 Hounsfield units (Fig. 1.1).
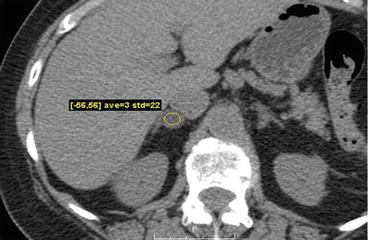

Fig. 1.1
Axial non-contrast CT scan demonstrates a hypodense nodule in the right adrenal gland with attenuation value of 3 Hounsfield units
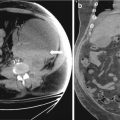
Axial non-contrast, contrast enhanced images at 1 and 15 min CT images from another patient demonstrate a right adrenal nodule with absolute washout of 67 % (Fig. 1.2a–c).
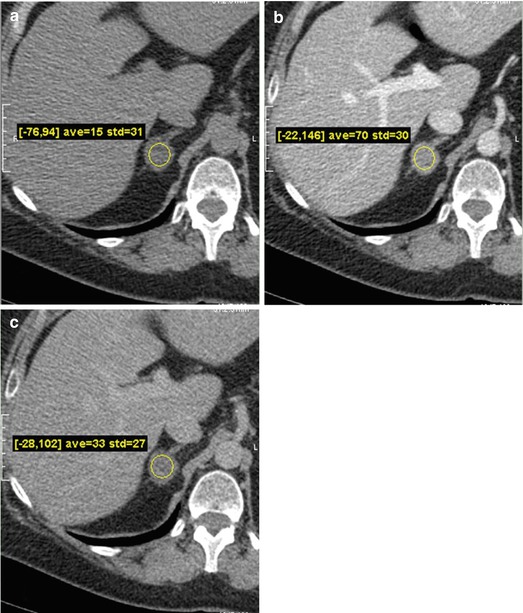

Fig. 1.2
(a–c). Axial non-contrast, contrast enhanced images at 1 and 15 min CT images from another patient demonstrate a right adrenal nodule with absolute washout of 67 %
Differential Diagnosis
Adrenal adenoma, pheochromocytoma, adrenocortical carcinoma, adrenal cyst, adrenal metastasis
Diagnosis and Discussion:
Adrenal adenoma
Adrenal adenomas are the most common primary benign adrenal neoplasm, typically found as an incidental finding. The prevalence increases with age, ranging from 0.14 % in patients 20–29 years up to 7 % in patients older than 70 years. Functioning adenomas which secrete cortisol (Cushing syndrome) or aldosterone (Conn syndrome) can occasionally be encountered. Although the majority of functioning adenomas will be unilateral, bilateral lesions can be seen in up to 20 % of patients.
Imaging findings typically demonstrate a well-defined, homogenous mass less than or equal to 3 cm. Histologically, the majority (ranging from 70 to 90 %) of adrenal adenomas will contain intracellular lipid. As a result, the majority of adrenal adenomas will demonstrate an attenuation value of less than 10 Hounsfield units on a non-contrast CT examination, and can be confidently diagnosed as a lipid rich adenoma. The remaining lesions (also known as lipid poor adenomas) may be further evaluated by examining their washout characteristics after the administration of intravenous contrast. The protocol generally consists of a non-contrast CT of the upper abdomen through the adrenal glands followed by contrast enhanced images at 60 s and delayed images at 15 min. Region of interests are drawn in the adrenal gland on all three phases of the examination and washout calculations are performed. An absolute washout of greater than 60 % or a relative washout of greater than 40 % accurately characterizes an adrenal adenoma. The presence of intracellular lipid in lipid-rich adenomas may be demonstrated on MR imaging evaluating for loss of signal intensity on opposed phase chemical shift imaging.
An incidental adrenal mass greater than or equal to 1 cm which is stable for over 1 year is generally considered to be benign and statistically likely to be an adenoma. Further evaluation with biochemical testing may be considered if there is clinical evidence for adrenal hyperfunction. No specific treatment or imaging follow-up is required for non-functioning adenomas. Surgical evaluation should be considered for functioning neoplasms.
Washout formulas:
1.
Absolute washout: 

2.
Relative washout: 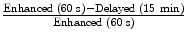
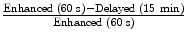
Pitfalls
Adrenal adenoma can be confidently diagnosed and differentiated using a combination of non-contrast imaging, washout calculations, and chemical shift MRI techniques. However, if non-contrast CT demonstrates an attenuation value of >10 HU, chemical shift MR imaging is unlikely to characterise the lesion further as this is likely to be a lipid poor adenoma. Adrenal cysts are benign non-enhancing lesions that may mimic adenomas on non-contrast imaging. Malignant lesions, such as adrenocortical carcinoma or metastasis are generally more heterogeneous and do not rapidly washout on delayed phase imaging. Pheochromocytomas are generally hypervascular lesions with variable high T2 signal. Washout characteristics are variable and these lesions rarely contain intracellular lipid, mimicking an adrenal adenoma. Clinical and laboratory evaluation of catecholamine excess may be considered, followed by functional imaging using 1–123 or I-131 MIBG imaging.
Teaching Point
1.
Adrenal adenomas are benign lesions that can be confidently diagnosed and differentiated using a combination of non-contrast imaging, washout calculations, and chemical shift MRI techniques.
2.
Asymptomatic lesions generally require no follow-up, however, adrenal adenomas may be hormonally active with subclinical features. Hormonally active tumors should be surgically evaluated.
3.
An incidental adrenal mass ≥1 cm which is stable for over 1 year is generally considered to be benign and statistically likely to be an adenoma. An incidental solid adrenal mass >4 cm without specific benign features (such as macroscopic fat to suggest a myelolipoma) should be surgically evaluated as most benign lesions are smaller in size (<3 cm).
Case 1.2
Brief Case Summary:
55 year old female with abdominal pain
Imaging Findings
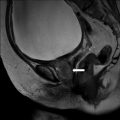
Coronal CT scan of the abdomen and pelvis with intravenous and oral contrast demonstrates a heterogeneous right suprarenal mass with areas of enhancing soft tissue as well as low attenuating regions compatible with underlying necrosis (Fig. 1.3).
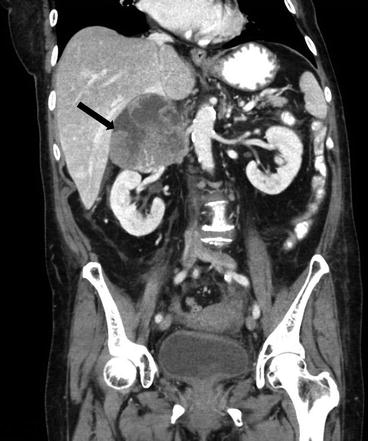

Fig. 1.3
Coronal CT scan of the abdomen and pelvis with intravenous and oral contrast demonstrates a heterogeneous right suprarenal mass (arrow) with areas of enhancing soft tissue as well as low attenuating regions compatible with underlying necrosis
Coronal CT scan of the abdomen and pelvis with intravenous and oral contrast in a different patient demonstrates a heterogeneous right suprarenal mass with extension into the liver parenchyma (Fig. 1.4).
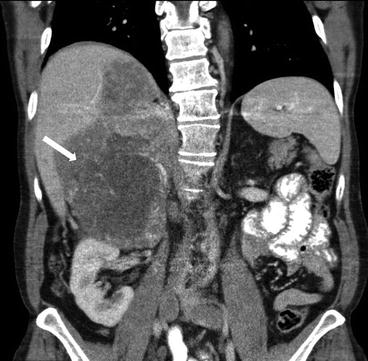

Fig. 1.4
Coronal CT scan of the abdomen and pelvis with intravenous and oral contrast in a different patient demonstrates a heterogeneous right suprarenal mass (arrow) with extension into the liver parenchyma
Axial CT scan of the abdomen and pelvis with intravenous and oral contrast in a third patient demonstrates a heterogeneous right retroperitoneal mass with extension into the inferior vena cava (Fig. 1.5).
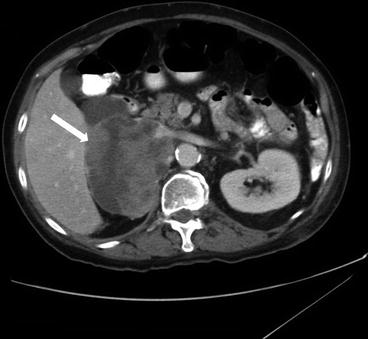

Fig. 1.5
Axial CT scan of the abdomen and pelvis with intravenous and oral contrast in a third patient demonstrates a heterogeneous right retroperitoneal mass (arrow) with extension into the inferior vena cava
Differential Diagnosis
Adrenal gland neoplasm (metastasis, adrenocortical carcinoma, pheochromocytoma), renal cell carcinoma, primary retroperitoneal neoplasm (such as a leiomyosarcoma).
Diagnosis and Discussion:
Adrenocortical carcinoma
Adrenocortical carcinoma (ACC) is a rare and aggressive neoplasm with a bimodal age of distribution, most often occurring before the age of 5, or within the third to fourth decades of life. Smoking and the use of oral contraceptives before the age of 25 are risk factors for developing ACC. The majority of ACCs are sporadic though some can be associated with genetic syndromes such as Li-Fraumeni syndrome and Carney complex. Clinically, patients may be asymptomatic or present with non-specific symptoms such as vague abdominal pain and nausea related to mass effect from the tumor. A subset of these tumors are hormonally active, with patients most commonly presenting with signs of Cushing syndrome or rapidly progressive virilization/feminization features.
ACCs are typically large at presentation, with most lesions measuring larger than 6 cm. The mass is heterogeneous with central areas of low attenuation corresponding pathologically to areas of necrosis. Approximately one-third of these tumors will demonstrate calcifications, typically centrally located. Contrast enhancement is often inhomogeneous with frequent retention of intravenous contrast material. Invasion of the inferior vena cava is seen in 9–19 % of patients on presentation. MRI demonstrates a heterogeneous mass with variable areas of T1 signal due to underlying hemorrhage as well as focal regions of high T2 signal related to underlying necrosis.
Open surgical resection of the neoplasm for localized neoplasm offers the best overall prognosis. For locally advanced or metastatic disease, chemotherapy with or without radiation is the standard, though primary debulking surgery of hormonally active neoplasm may be considered to alleviate symptoms. The overall prognosis is poor with 5 year survival rate of 38 %. Local recurrence and metastatic disease are common with one third of patients demonstrating metastatic disease on presentation.
Pitfalls
Common mimickers of ACC include adrenal metastases, pheochromocytoma, and adrenal adenoma. Adrenal metastases should be considered if there is a known primary with metastatic disease elsewhere, particularly if there are bilateral adrenal masses. Adrenal adenoma can be distinguished by their attenuation values on non-contrast CT exams and their rapid washout after the administration of contrast. Pheochromocytomas may present with signs of catecholamine excess and will often demonstrate uptake on MIBG scintigraphy. Distinguishing an ACC from a renal cell cancer or primary retroperitoneal sarcoma can be done by observing the mass effect on surrounding structures. Coronal and sagittal reformatted images are often helpful in this regard.
Teaching Points
1.
ACCs are large, heterogeneous neoplasms with areas of necrosis and calcifications. Prognosis is poor and complete surgical resection offers the best overall prognosis.
2.
IVC invasion is frequent on initial presentation. Although IVC invasion does not preclude surgical resection, accurate reporting of this is imperative for surgical planning.
3.
A solid adrenal mass greater than 4 cm, despite benign imaging features, is generally managed as a malignant lesion. In addition, distinguishing an ACC from a non-functioning pheochromocytoma without MIBG uptake may be impossible without surgical resection.
Case 1.3
Brief Case Summary:
34 year old female, incidental adrenal mass on CT scan of the chest.
Imaging Findings
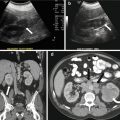
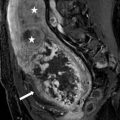
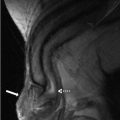
A CT scan of the chest performed with intravenous contrast demonstrates an incompletely imaged, low attenuating left adrenal mass with a single peripheral punctate calcification (Fig. 1.6). Gray scale and color images of the left kidney demonstrate the presence of hypoechoic left adrenal mass (Fig. 1.7a) without detectable flow (Fig. 1.7b). Non-contrast MRI evaluation demonstrates the presence of a T2 hyperintense left adrenal mass (Fig. 1.8) without loss of signal on the opposed phase images (Fig. 1.9).
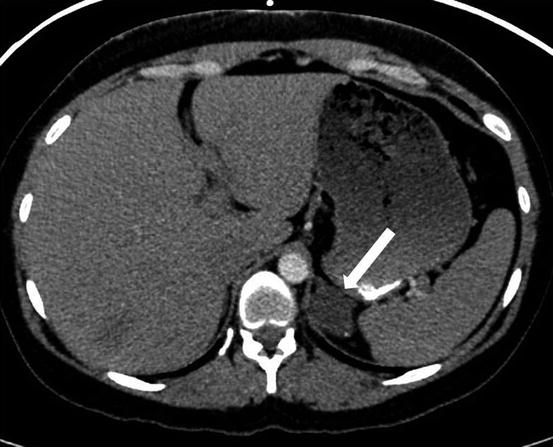
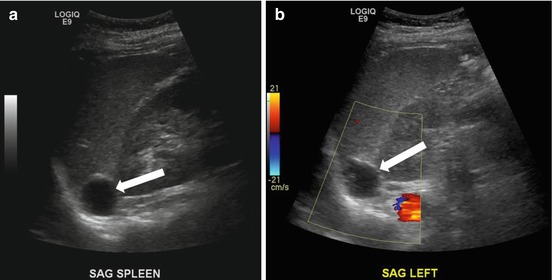
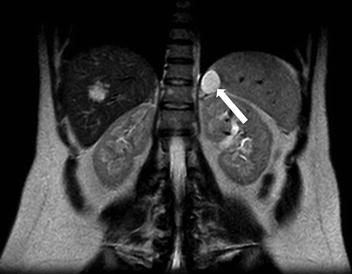
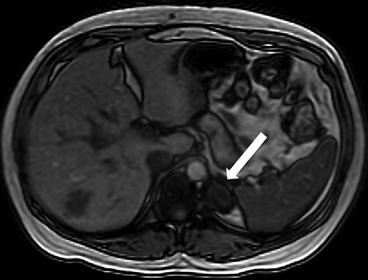

Fig. 1.6
CT scan of the chest performed with intravenous contrast demonstrates an incompletely imaged, low attenuating left adrenal mass (arrow) with a single peripheral punctate calcification

Fig. 1.7
Gray scale and color images of the left kidney demonstrate the presence of hypoechoic left adrenal mass (arrow) (a) without detectable flow (b)

Fig. 1.8
Non-contrast MRI evaluation demonstrates the presence of a T2 hyperintense left adrenal mass (arrow)

Fig. 1.9
Non-contrast MRI evaluation demonstrates the presence of a left adrenal mass (arrow) without loss of signal on the opposed phase images
Differential Diagnosis
Pheochromocytoma, adrenal adenoma, adrenal metastases, adrenal cyst
Diagnosis and Discussion:
Adrenal cyst (lymphangioma)
Adrenal cysts are uncommon lesions and are histologically classified as endothelial cysts, pseudocysts, epithelial cysts, and parasitic cysts, in decreasing order of frequency. Endothelial cysts include lymphangiomatous (more common) and angiomatous types, while pseudocysts are thought represent the sequelae of hemorrhage related to an underlying mass, trauma, or infection. Adrenal cysts are most often discovered as incidental masses, though they may occasionally grow large enough to cause non-specific symptoms related to mass effect. In addition, adrenal cysts can be associated with a variety of syndromes including polycystic renal disease, Beckwith-Wideman syndrome and Klippel-Trenaunay-Weber syndrome. Cystic metastases or a primary cystic endocrine tumor, are important mimickers of benign adrenal cysts.
Radiological differentiation of the subtypes of benign adrenal cysts is challenging. Endothelial cysts are typically non-enhancing masses with thin smooth walls with an internal density value of less than 20 Hounsfield units. A multilocular appearance with thin septations and occasional septal calcifications has also been described. On the other hand, pseudocysts typically manifest as unilocular cystic masses with a variable degree of wall thickness (often up to 5 mm) and mural calcification. Internal hemorrhage within the pseudocyst may give a complex appearance, though evolution of the internal appearance over time due to break down of blood products and the lack of enhancement will be noted. Epithelial cysts manifest as simple appearing cysts on both CT and MR imaging with fluid signal, thin walls, and no enhancement after contrast administration. Parasitic cysts are most often secondary to echinococcus with imaging demonstrating the presence of septal and mural calcifications as well as the presence of internal floating membranes and daughter cysts.
Management of adrenal cysts is controversial. Further evaluation is based on determining the functional status of the mass to exclude a cystic endocrine neoplasm and imaging features to exclude the possibility of a cystic degeneration of solid mass. In addition, some authors advocate the resection of any benign appearing adrenal cyst >5 cm due to the potentially increased risk of hemorrhage and symptoms from adjacent mass effect.
Pitfalls
Adrenal cysts with internal hemorrhage can often have a complex CT and sonographic imaging appearance, potentially mimicking an adrenal cortical carcinoma or metastases. MRI is more useful in these circumstances, given the better contrast resolution of soft tissues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree