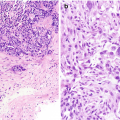
Cytology of normal renal tissue . (a) Proximal renal tubule, Diff-Quik stain, 400×. (b) Glomerulus and proximal renal tubule, Diff-Quik stain, 400×
Glomeruli
Consist of large clusters of capillary loops and mesangial cells that are surrounded by Bowman capsule (Fig. 5.1b).
Appropriate identification of this normal structure is critical so as to not misinterpret them as tumor cells.
Renal Cysts
Clinical
Very common.
Possibly congenital or acquired, single or polycystic, non-neoplastic or neoplastic, and benign or malignant.
Cytology of FNA or Touch Preparation of Core
Benign cysts: yield clear or pale, yellow fluid with few small, bland, or mildly atypical epithelial lining cells in small three-dimensional clusters; macrophages are seen in most cases. Additionally, neutrophils and occasionally Liesegang rings (three-dimensional laminated ring-like acellular structures) are also observed [5–7]. Epithelial cells seen within these benign specimens demonstrate round nuclei and ill-defined cell borders.
Malignant cysts: generally bloody or “chocolate”-like fluid and the presence of atypical epithelial cells, macrophages, and lymphocytes [7]. Fluid from cystic renal cell carcinoma (RCC) may show features similar to those of benign cysts [7].
Comments
- 1.
Benign epithelial cells with reactive atypia, especially with vacuolated cytoplasm; should be distinguished from RCC with cystic degeneration or cystic RCC.
- 2.
Proximal renal tubule cells should be distinguished from oncocytoma or chromophobe type RCCs [8].
- 3.
Chronic interstitial inflammation: Inflammatory changes can be seen in the kidneys, and the resultant distribution and nonspecific imaging characteristics may appear more nefarious in nature.
- 4.
Renal infection: Pyelonephritis may result in wedge-shaped areas of hypoenhancement in the renal parenchyma. Renal abscesses demonstrate findings of abscesses elsewhere in the body, including a central area of fluid with an enhancing rim. On MRI, these restrict diffusion.
Benign Renal Neoplasms
Angiomyolipoma
Angiomyolipoma (AML) is a benign mesenchymal tumor composed of a variable proportion of adipose tissue, spindle and epithelioid smooth muscle cells, and abnormal thick-walled blood vessels. Angiomyolipomas are the most common benign tumors of the kidney and are more prevalent in the female population [9, 10].
Clinical
Overall an uncommon entity, thought to represent 0.5–2% of all renal tumors. Some angiomyolipomas are associated with tuberous sclerosis [11–13].
Male-to-female ratio is 1:4 to 1:10 in nontuberous sclerosis and 1:1 in tuberous sclerosis [11–13]. Average age is 45–55 years for patients without tuberous sclerosis and 25 to 35 for those patients with tuberous sclerosis [11–13].
Usually asymptomatic. Patients may present with flank pain, hematuria, palpable mass, and retroperitoneal hemorrhage.
Radiology
On MRI, it is challenging to distinguish the microscopic fat of AMLs from that of clear cells on in-phase and out-of-phase T1-weighted imaging (Fig. 5.2) [14]. In addition, AMLs with lower lipid content are difficult to distinguish from papillary RCCs on T2-weighted images.
Though AMLs are the most common benign tumor, there are abundant imaging pitfalls. Of note, if AML is >4 cm, prophylactic embolization is recommended because of the risk of rupture. Angiographically, AMLs have high vascularity and appear different from surrounding renal parenchyma.
Angiomyolipoma , radioimages. (a) Axial SHARP MRIs show a lesion in the right kidney that is isointense to the adjacent renal parenchyma. (b) Axial SHARP MRI at 17-minute delay shows a right renal lesion that is hypointense to renal parenchyma. (c) Axial SHARP postcontrast MRI sequence showing minimally increased ROI value consistent with enhancement
Cytology of FNA and Touch Preparation of Core
Characteristically demonstrate a variable proportion of blood vessels and spindle and epithelioid smooth muscle spindled cells arranged in loosely cohesive or cohesive clusters as well as mature fat cells (Fig. 5.3) [15]. The nuclei of smooth muscle cells are oval to elongated with evenly distributed chromatin and no or inconspicuous nucleoli [15]. Naked nuclei are common. The cytoplasm is delicate and sometimes finely vacuolated [16]. Occasionally, smooth muscle cells and adipocytes may show atypia [15]. Mitoses and necrosis are absent [15]. Adipose tissue is not universally present [15].
Fat necrosis can be seen, including histiocytes, multinucleated giant cells, and pleomorphic nuclei of adipocytes [17].
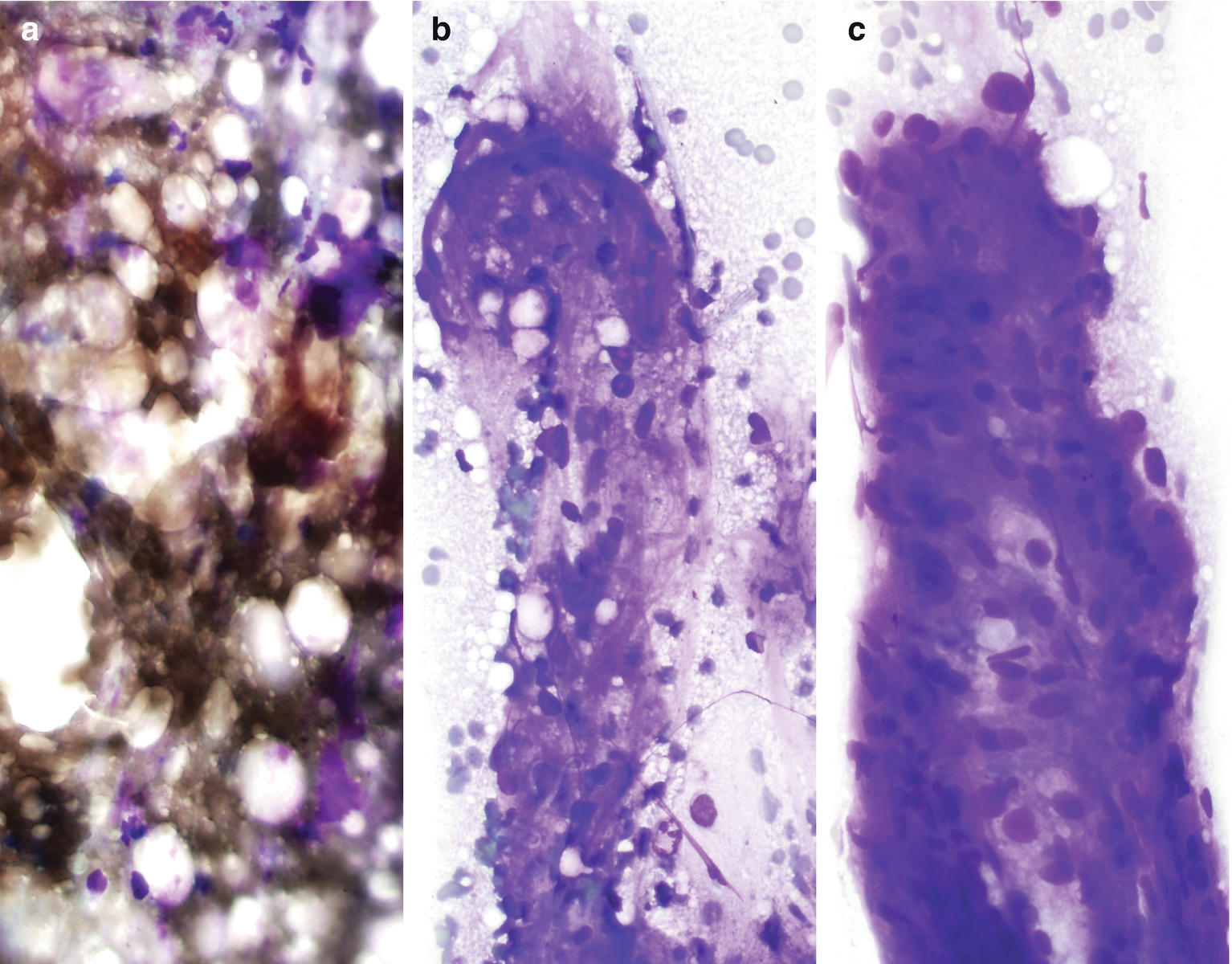
Angiomyolipoma , cytology. (a–c) FNA biopsy of a renal mass, Diff-Quik stain, 400×
Comments
Differential diagnosis includes perinephretic fat (misinterpreted as tumor adipose tissue), RCC (misinterpreted as epithelioid or atypical spindle smooth muscle cells), leiomyosarcoma (misinterpreted as atypical smooth muscle cells), and liposarcoma (misinterpreted as atypical adipocytes).
These lesions are often sparely cellular, and positive staining of tumor cells can be very helpful in definitive diagnosis. Tumor cells are positive for melanocytic markers (HMB-45, CD63, tyrosinase, and Mart1/Melan-A), smooth muscle markers (smooth muscle actin, muscle-specific actin, desmin, and calponin), CD68, neuron-specific enolase, S-100, estrogen receptor, and progesterone receptor.
Oncocytoma
Oncocytoma is a benign renal epithelial neoplasm composed of large cells with mitochondria-rich eosinophilic cytoplasm; it is thought to arise from intercalated cells.
Clinical
Approximately 3–7% of all renal neoplasms [18, 19].
Wide age range, with a peak in the seventh decade. Male-to-female ratio is 2:1 [20].
Mostly asymptomatic, and a small number of patients present with hematuria, abdominal pain, and flank mass.
Central stellate fibrous scar.
Can be bilateral and multicentric and solid or cystic.
Radiology
Oncocytoma , radioimages. (a) CT scan shows a hyperenhancing mass in the middle pole of the right kidney which contains a central region of low density that may represent necrosis. (b) Coronal CT scan images show a hyperenhancing mass with a central region of necrosis
Cytology of FNA and Touch Preparation of Core
Characteristically show single or small loose clusters of monotonous oncocytes (Fig. 5.5) [22].
Oncocytes are large, polygonal or rounded with abundant and finely granular cytoplasm with well-defined cell borders [22].
The nuclei are usually small, round, regular, and centrally or eccentrically located (Fuhrman grade 2), with finely granular chromatin and no to conspicuous nucleoli [22, 23]. However, hyperchromatic or bizarre nuclei, binucleation, and nuclear enlargement can also be seen and are likely thought to represent degenerative change. Mitosis and necrosis are not usually observed.
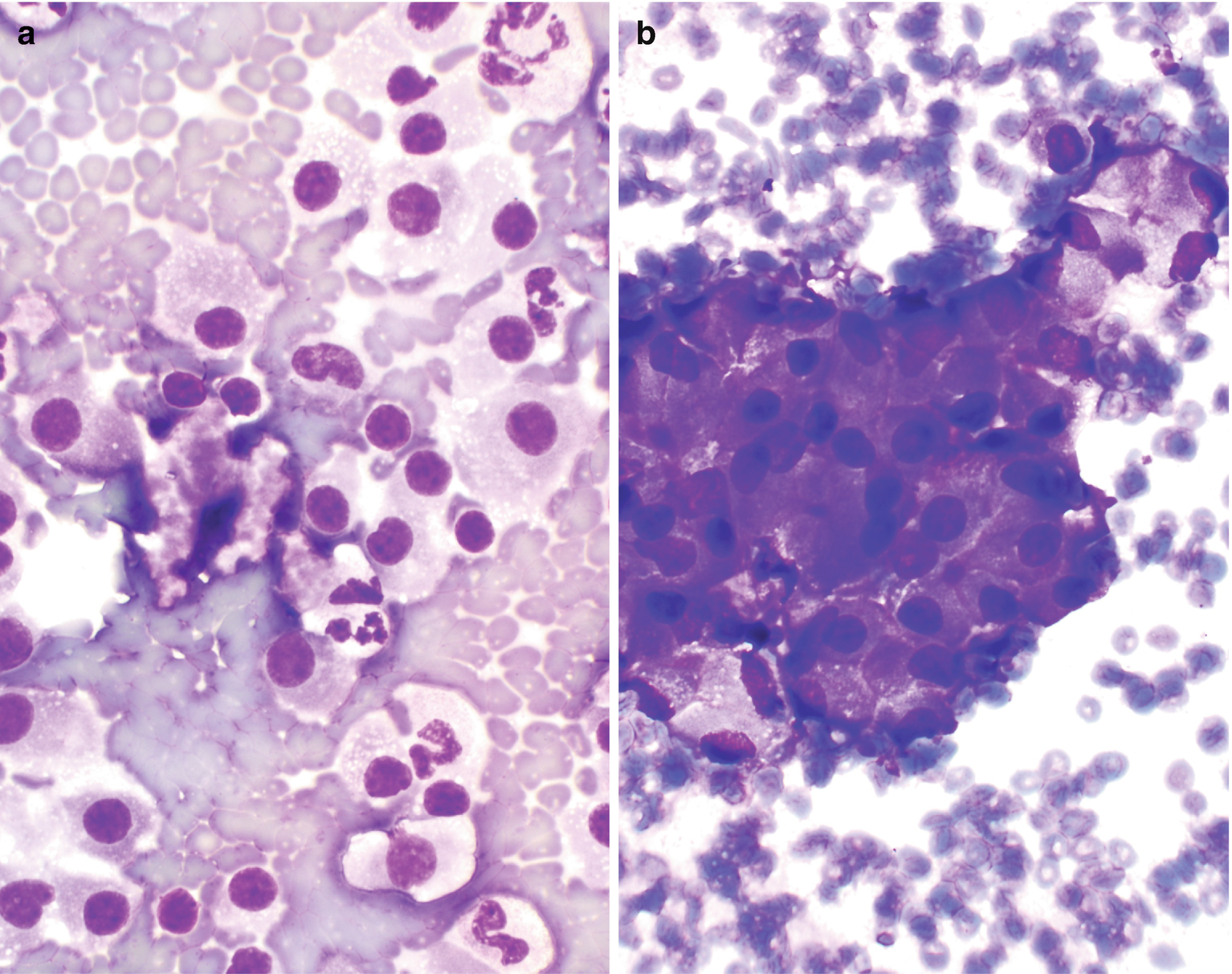
Oncocytoma , cytology. (a, b) FNA biopsy of a renal mass, Diff-Quik stain, 600×
Comments
The differential diagnosis includes chromophobe RCC , clear cell RCC, normal renal tubular cells, and normal hepatocytes.
The tumor cells are positive for CK7, epithelial membrane antigen (EMA), CD117, napsin A [24], E-cadherin, and S-100A1; they are less frequently positive for CD10 and alpha-methylacyl-CoA racemase (AMACR) [25]. Tumor cells are negative for the RCC antigen, vimentin [25]. There is no diffuse cytoplasmic Hale colloidal iron staining.
Malignant Renal Tumors
Renal Cell Carcinoma
RCC is a group of malignancies arising from the epithelium of the renal tubules. Based on the WHO classification (2016), it consists of familial renal cancer, clear cell RCC, multilocular cystic RCC, papillary RCC, chromophobe RCC, carcinoma of the collecting ducts of Bellini, renal medullary carcinoma, renal carcinomas associated with Xp11.2 translocations/TEF3 gene fusions, and clear cell papillary RCC [26]. Other tumors include RCC associated with neuroblastoma, mucinous tubular spindle cell carcinoma, papillary adenoma of the kidney, and unclassified RCC [27].
The clear cell subtype of RCC has worse outcomes relative to chromophobe and papillary subtypes, with a greater likelihood of metastasizing and presenting at a later stage [27–30]. The chromophobe subtype of RCC is less frequently seen than clear cell RCC and papillary RCC, with an incidence of approximately 5%. The papillary subtype of RCC is often discovered at a low grade stage and is small in size [30]. The size used to separate papillary RCC from papillary adenoma was increased from 0.5 cm to 1.0 cm in the 2016 WHO classification [27]. The five-year survival rate of papillary RCC is significantly better than that for clear cell, ranging from 82% to 92% [31, 32].
Clinical
The ninth most common cancer in men and the fourteenth most common cancer in women [33] and >90% of all malignancies of the kidney [33].
Male-to-female ratio is 2 to 3:1 [33, 34]. Its incidence increases steadily after 40 years of age [33].
Clinical triad: hematuria, pain, and flank mass. Paraneoplastic endocrine syndromes can be seen [35].
Can be solitary or cystic and single or multiple.
Radiology
RCC, clear cell type : It typically exhibits heterogeneity caused by necrotic change, hemorrhage, or cystic change [32, 36]. It also exhibits features typical of most hypervascular lesions on CT (Fig. 5.6) [30].
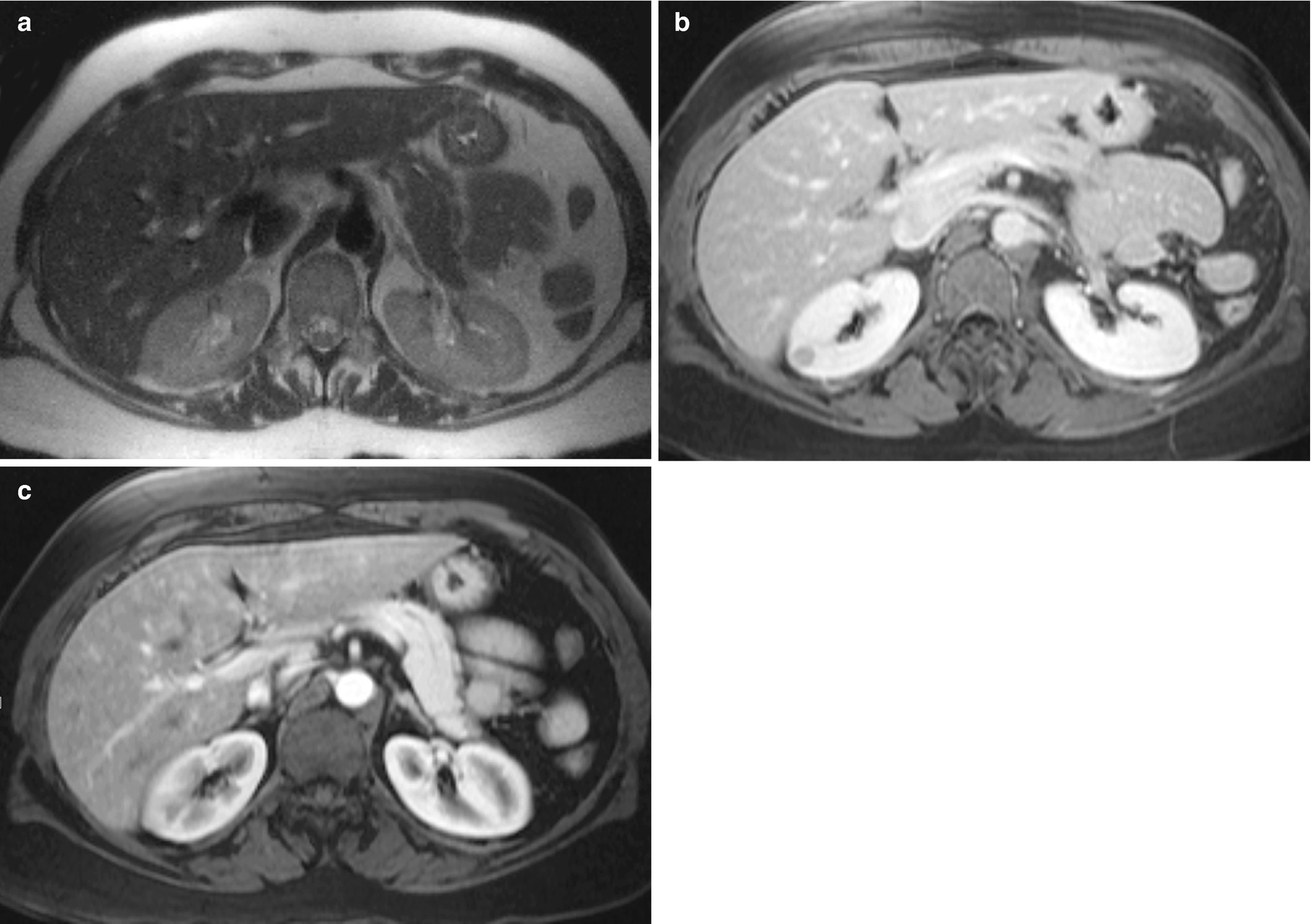
RCC, chromophobe type : The CT scan of chromophobe RCC shows a solid, homogeneous appearance with possible spoke-wheel enhancement or a stellate central scar (Fig. 5.7) [30].
RCC, papillary type : The papillary subtype of RCC exhibits low signal intensity on T2-weighted MRI and is also hypervascular (Fig. 5.8).
RCC, sarcomatoid differentiation: The sarcomatoid differentiation can be found in any subtype of RCC. Thus, imaging features would probably be reflective of the subtype, although with aggressive features and resultant poor prognosis as shown in the CT scan (Fig. 5.9) [30].
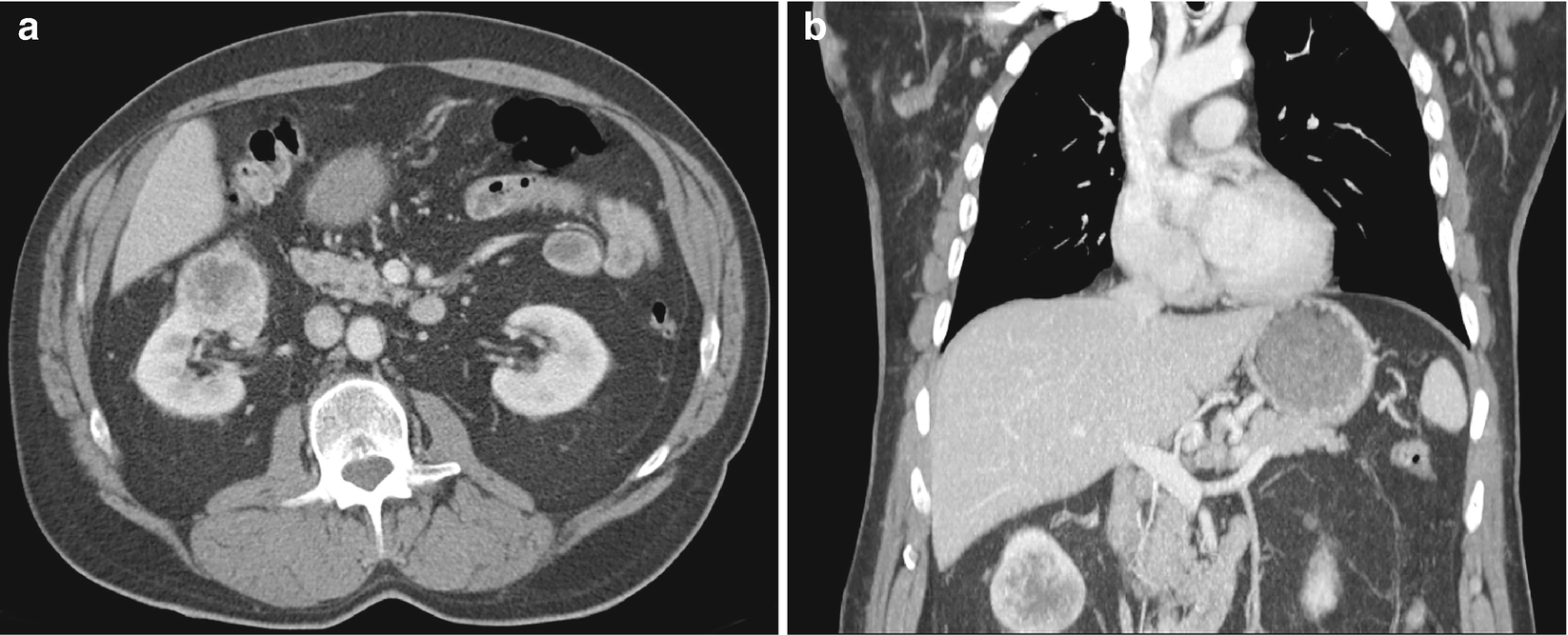
RCC, clear cell type, radioimages. (a) CT scan shows an exophytic mass arising from the inferior pole of the right kidney. There is evidence of central necrosis. (b) CT scan on coronal view, in which the mass appears to have a central region of necrosis
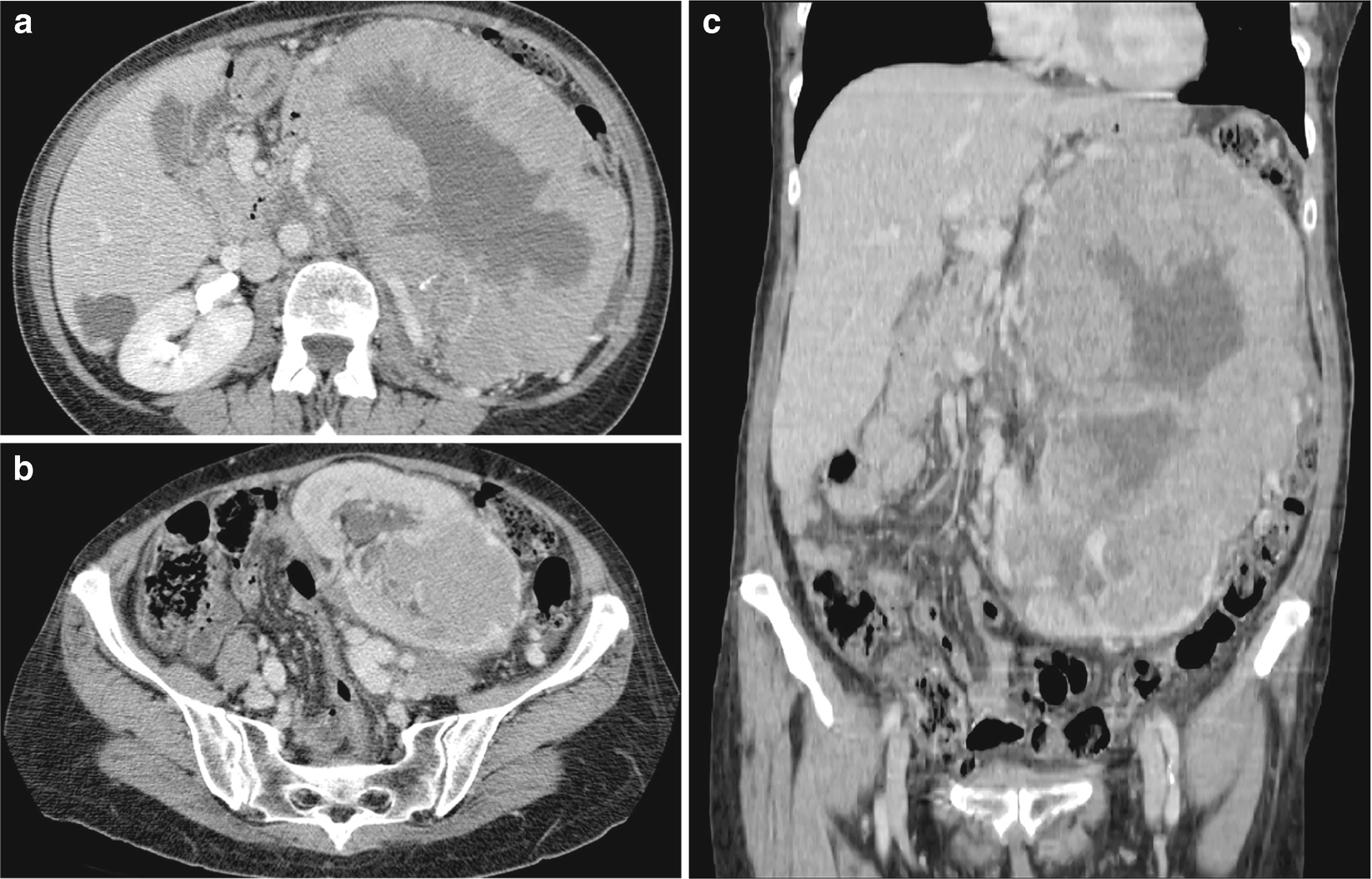
RCC, chromophobe type, radioimages. (a) CT scan shows a large, heterogeneously enhancing multilobulated mass arising from the left kidney. Low density regions may represent necrosis or poorly enhancing tissue. There are coarse calcifications within the mass. (b) CT scan shows another view farther inferior to the large mass with associated compression and displacement of adjacent vessels. Hypoattenuating regions are again seen, possibly caused by necrosis. (c) CT scan shows that the true extent of the mass can be appreciated on the coronal image
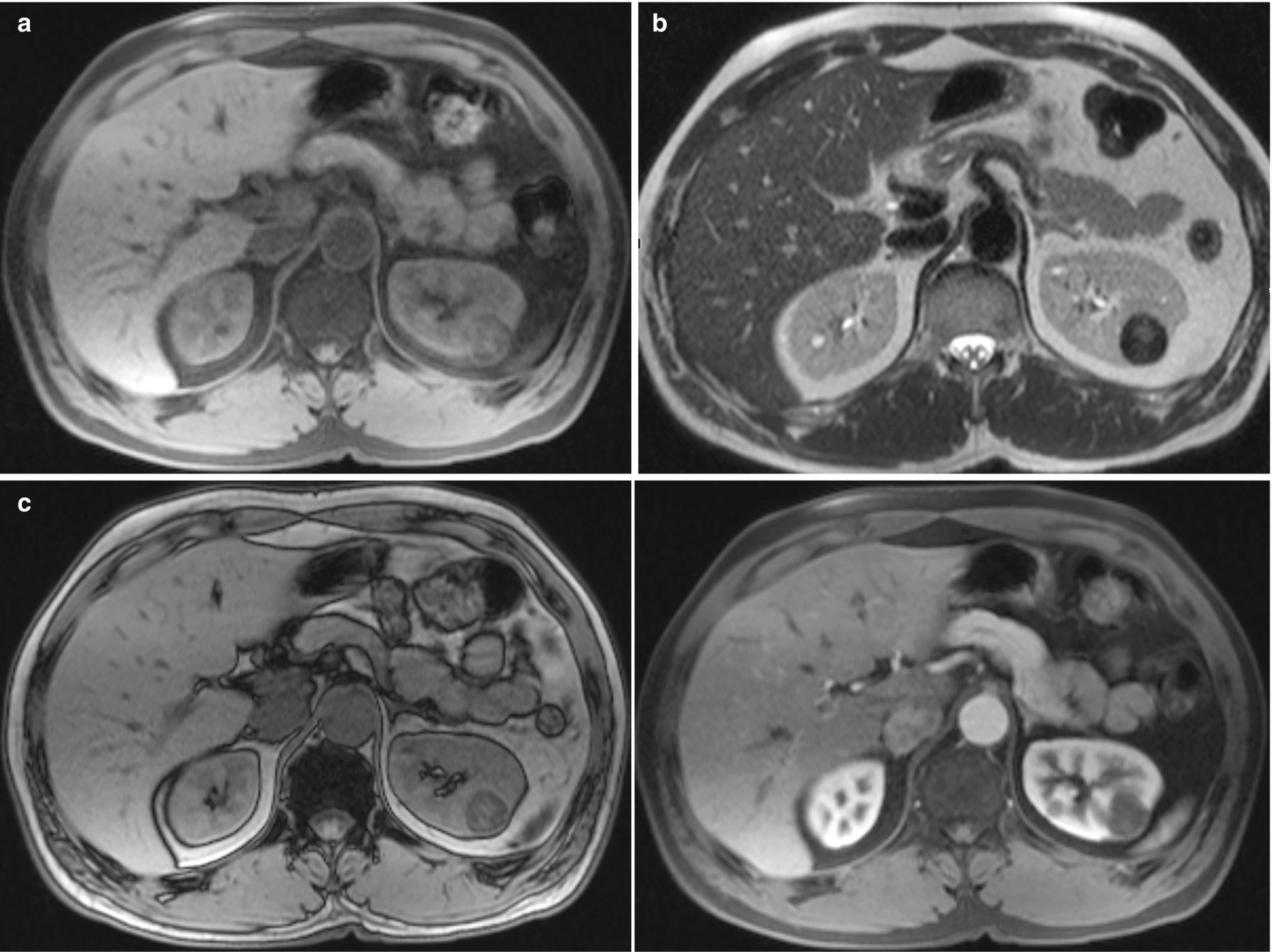
RCC, papillary type, radioimages. (a) MRI (axial T1) shows a 2.5-cm lesion in the superior pole of the left kidney, which is slightly heterogeneous and showing decreased intensity on T1-weighted images. (b) MRI (axial HASTE) shows a hypointense lesion in the left kidney. (c) MRI (in-phase image) shows signal loss when compared to the out-of-phase images. (d) MRI (postcontrast) sequence of the lesion demonstrates mild early enhancement, which increases slowly over time
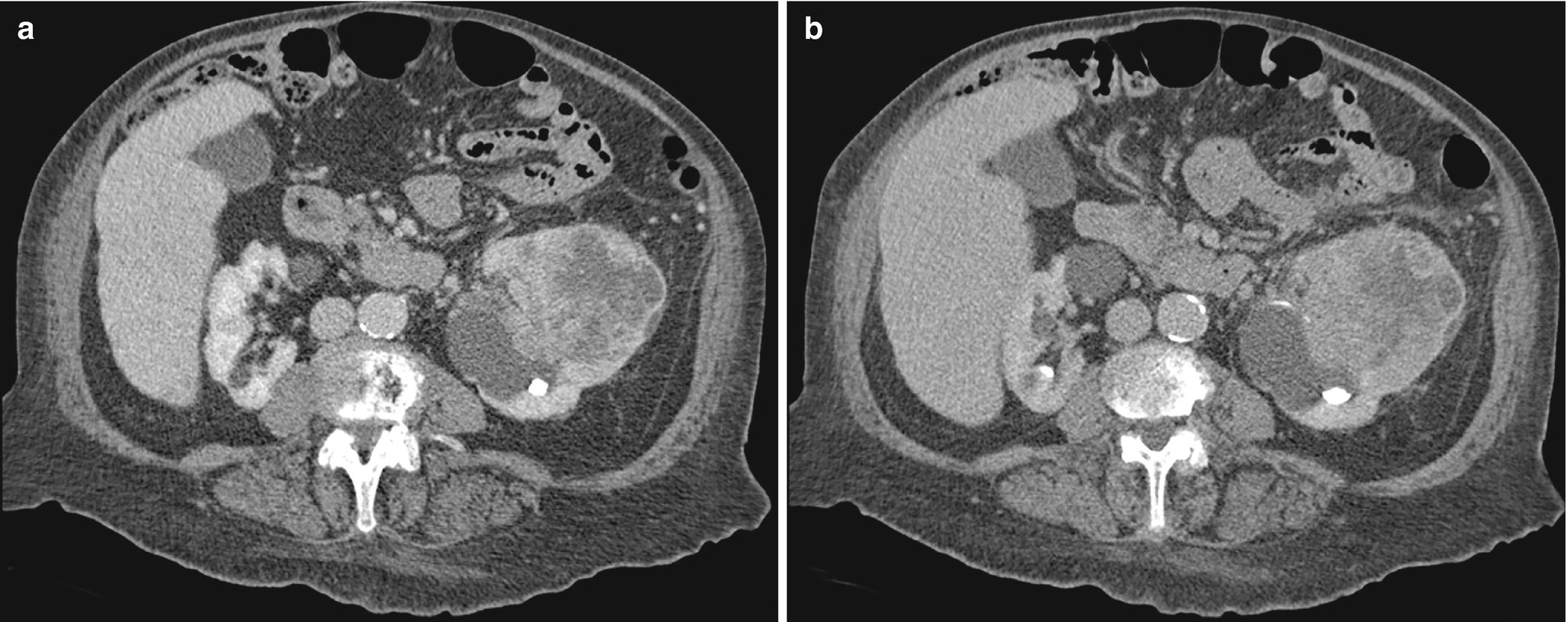
RCC with sarcomatoid differentiation, radioimages. (a) CT scan shows a large, peripherally enhancing heterogeneous mass with suspected invasion of the proximal ureter as well as possible thrombosis of a renal vein branch. (b) CT again shows a large, heterogeneous mass obscuring the left upper pole and a portion of the renal pelvis
Cytology of FNA and Touch Preparation of Core
RCC, clear cell type : The most common cell type. Characteristically shows single, sheets, nests, papillary or floral-like groups, or three-dimensional clusters of clear cells with or without capillaries transversing the cells (Fig. 5.10a, b). The clear cells are large with low nuclear/cytoplasmic ratios. The cytoplasm is abundant, pale, wispy, delicate, reticulated, foamy, vacuolated, or granular. The nuclei vary from small to large, uniform to pleomorphism, bland to bizarre, smooth to irregular nuclear membrane, fine and evenly distributed chromatin to coarse and unevenly distributed chromatin, and no nucleoli to prominent nucleoli, depending on the grade of the tumor [6]. Intranuclear cytoplasmic invaginations, intracytoplasmic hyaline globules, naked nuclei, and multinucleated giant tumor cells can also be seen [6]. Necrosis, frothy background, fibrosis, hemosiderin, cholesterol, and fat may be seen in the background [6].
RCC, chromophobe type : Characteristically shows clusters or single large cells with granular cytoplasm, well-defined cell borders, and a low-to-high nuclear/cytoplasmic ratio (Fig. 5.11a, b) [37]. The nuclei vary significantly in size, are often atypical, and have coarse and hyperchromatic chromatin, irregular nuclear membrane, smaller nucleoli, and peri-nuclear haloes [6]. Binucleation or multinucleation is frequently seen [6, 37]. The cells are often referred to as “vegetable cells.” Hemorrhage and necrosis are common, particularly in larger tumors.
RCC, papillary type : Characteristically shows papillae composed of fibrovascular stalks surrounded by a layer of tumor cells, rounded spherules of tumor cells, single cells, and foamy or hemosiderin-laden macrophages (Fig. 5.12a). The fibrovascular stalks often contain histiocytes, with or without hemosiderin and/or lipid. The tumor cells are usually cuboidal to columnar. The nuclei are usually bland and uniform with fine chromatin, smooth to irregular nuclear membrane, small or inconspicuous nucleoli, and high nuclear/cytoplasmic ratios [6]. Pleomorphism, multinucleation, and mitotic figures are infrequent. Cytoplasm is scant and clear/pale in type 1 and abundant/eosinophilic in type 2. The tumor cells often contain hemosiderin pigment and may also contain intracytoplasmic vacuoles. Psammoma bodies, necrosis, and hemorrhage can be seen.
RCC, with sarcomatoid differentiation : Characteristically shows discohesive spindle and pleomorphic cells with prominent nucleoli and delicate cytoplasm (Fig. 5.13a, b). Necrosis is frequently seen [16]. These cells show epithelial features by immunohistochemistry (IHC) and electron microscopy.
Collecting duct carcinoma : Characteristically shows single or clusters of round or oval cells with tubule or papillary architectures. The nuclei are large and mildly atypical and have hyperchromatic and coarse chromatin and inconspicuous to prominent nucleoli. The cytoplasm is small to moderate in amount and finely granular with well-defined cell borders [38].
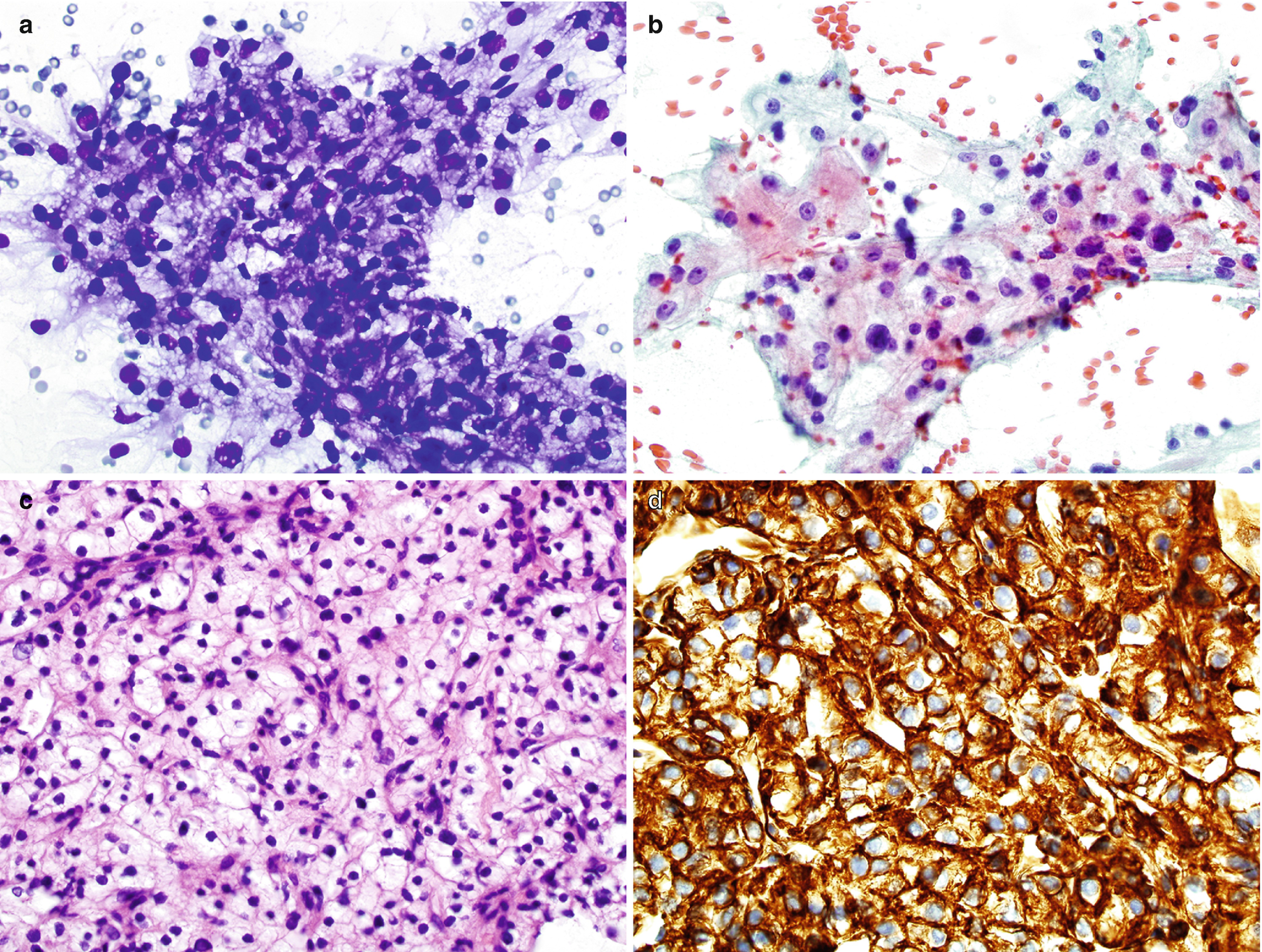
RCC, clear cell type, cytology and histology . (a, b) Biopsy cytology of a renal mass, Diff-Quik stain (a) and Papanicolaou stain (b), 400×. (c) Histology of core, hematoxylin and eosin stain, 400×. (d) Immunostain for CA9, 400×
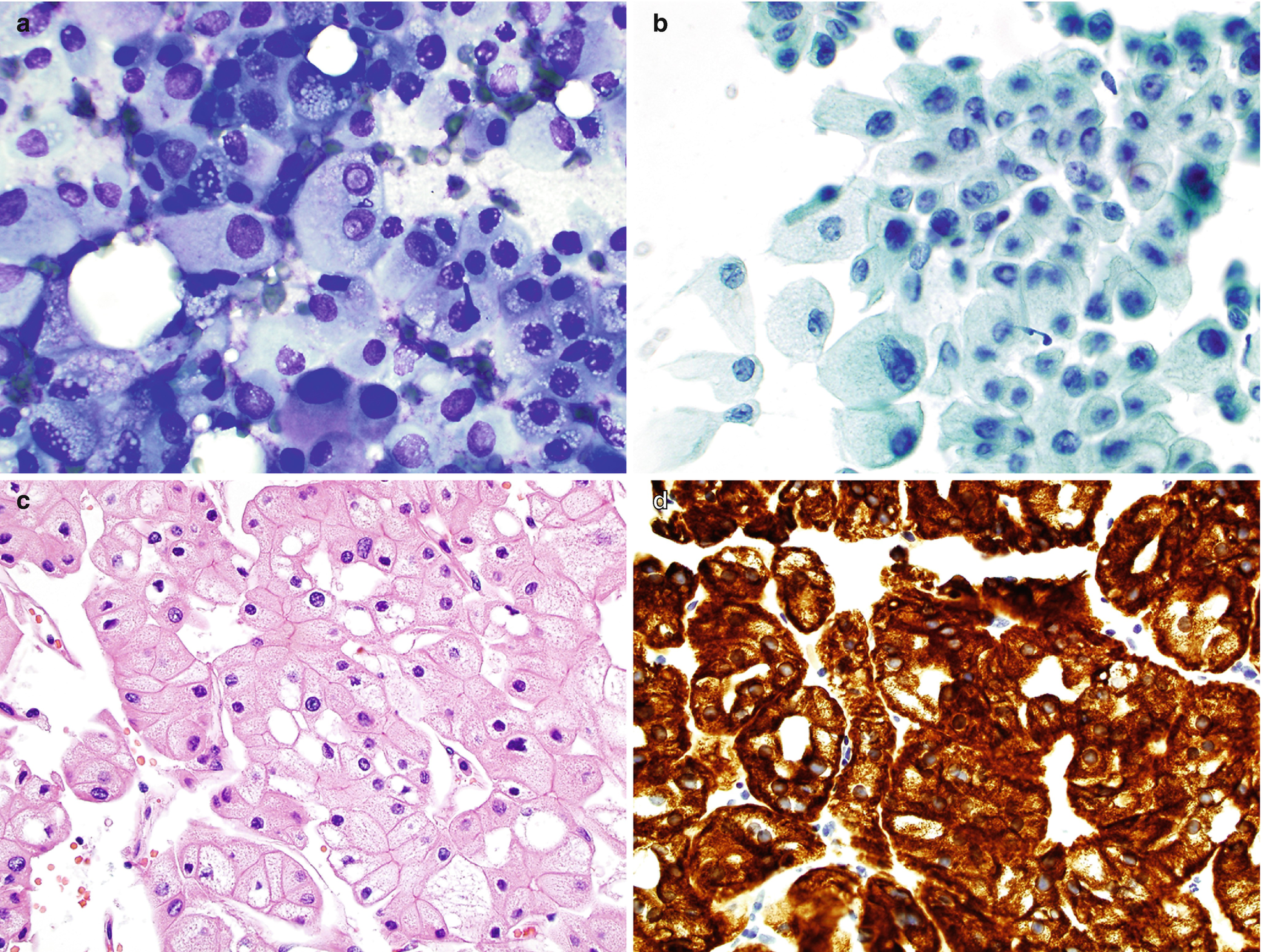
RCC, chromophobe type, cytology and histology. (a, b) FNA biopsy of a renal mass, Diff-Quik stain (a) and Papanicolaou stain (b), 400×. (c) Histology of core, hematoxylin and eosin stain, 600×. (d) Immunostain for CK7, 600×
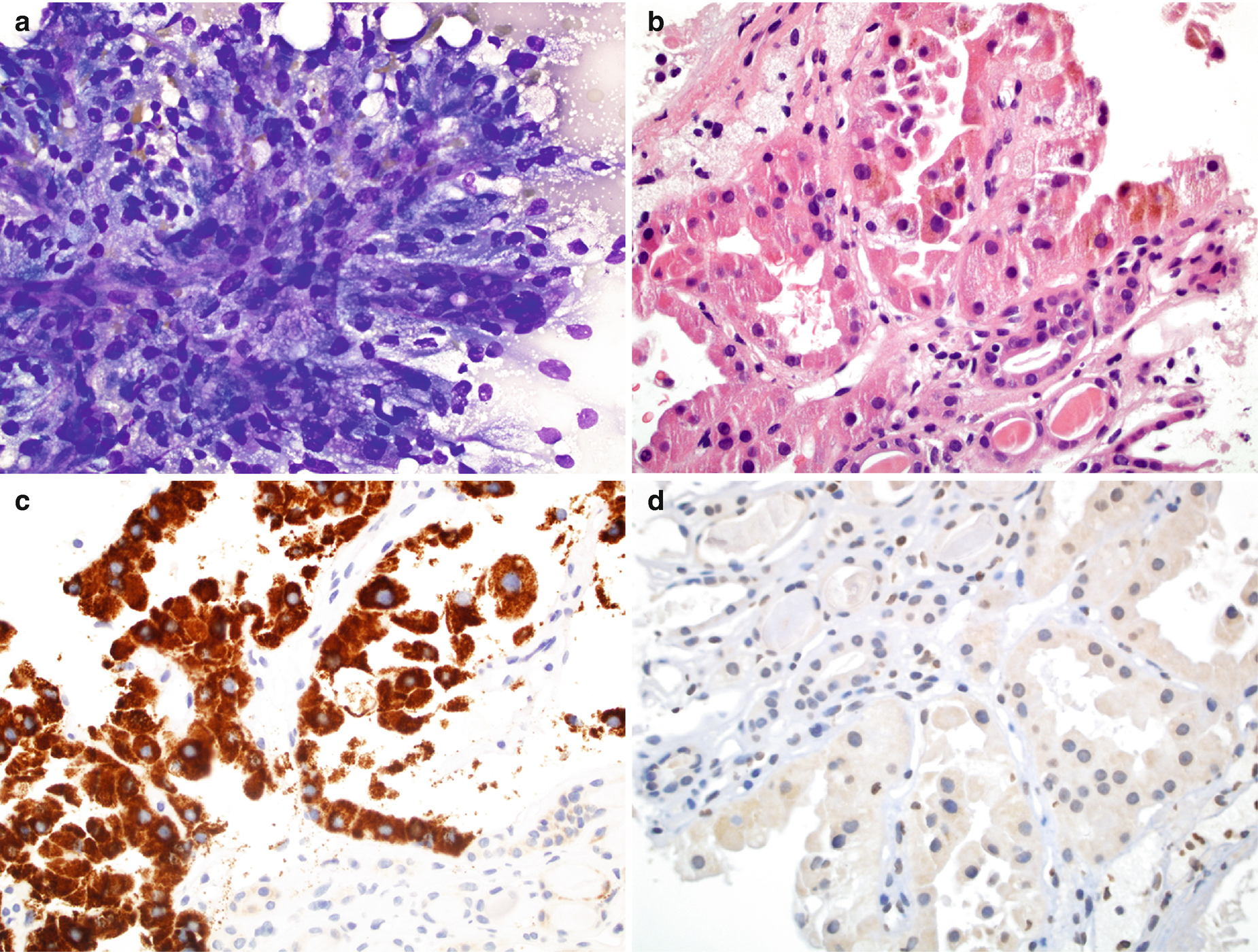
RCC, papillary type, cytology and histology . (a) FNA cytology of a renal mass, Diff-Quik stain, 400×. (b) Histology of core, hematoxylin and eosin stain, 600×. (c, d) Immunostain for AMACR (c) and CA9 (d) 600×
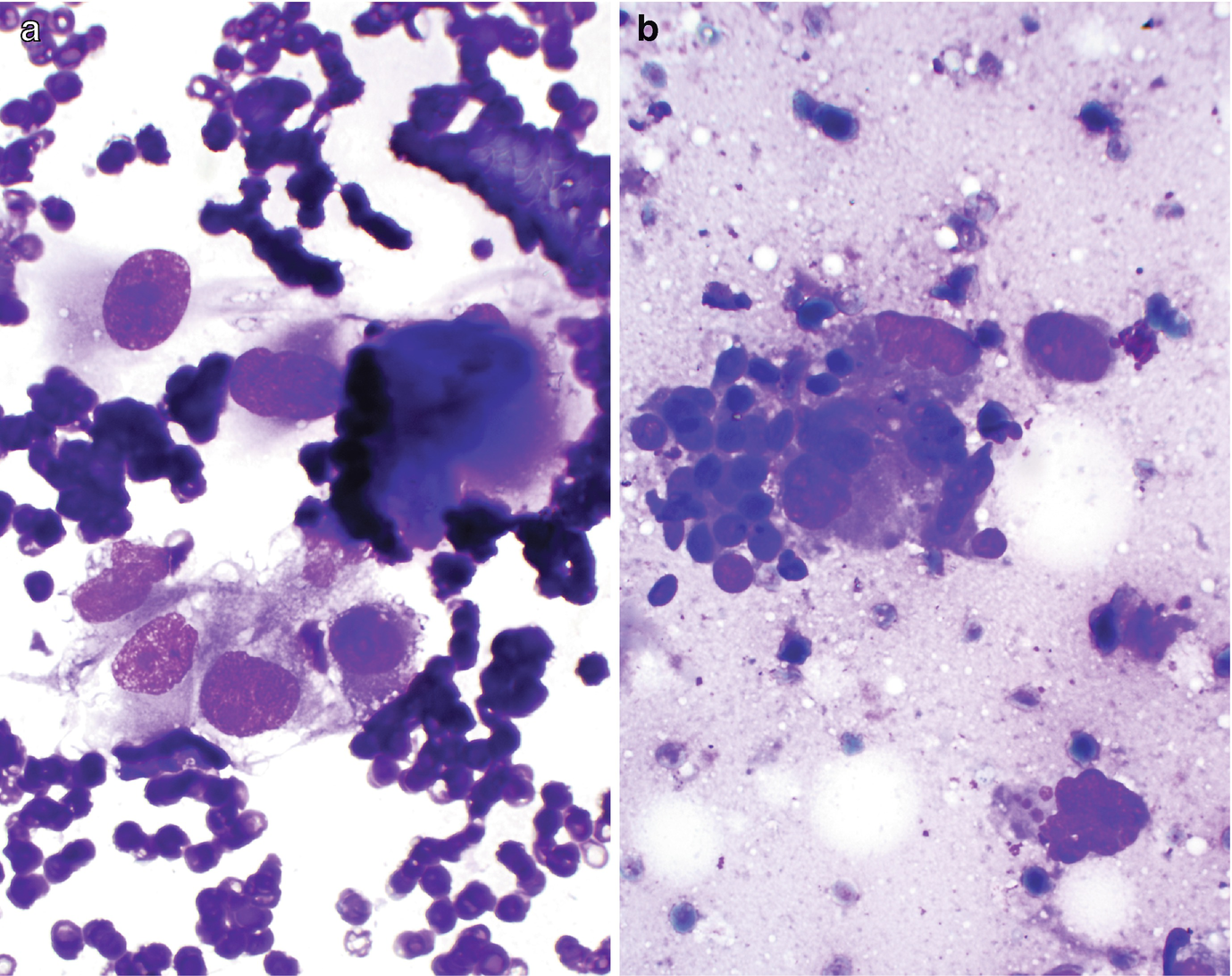
RCC, high grade, with sarcomatoid feature. (a, b) FNA biopsy of a renal mass, Diff-Quik stain, 600×
Histology of Core or Cell Block
RCC, clear cell type : Sections show nests or sheets of large polygonal cells with capillary networks, foamy, clear, or granular cytoplasm, and centrally located nuclei that possibly contain nucleoli, depending on grading (Fig. 5.10c).
RCC, chromophobe type : Sheets of polygonal cells with abundant granular cytoplasm, well-defined cytoplasmic borders, and round or oval nuclei with perinuclear halos (Fig. 5.11c).
RCC, papillary type : Papillae lined by atypical epithelial cells with nuclear atypia with or without prominent nucleoli, depending on grading of tumor; scant to abundant eosinophilic, granular, or vacuolated cytoplasm, depending on type of papillary RCC (type 1 versus type 2) (Fig. 5.12b).
Comments
For clear cell RCC, the differential diagnosis includes chromophobe RCC, papillary RCC, clear cell papillary RCC histiocytes [39], degenerated tubular cells, benign adrenal cortical cells, adrenocortical neoplasms, metastatic clear cell carcinoma from other primary tumors, and angiomyolipoma. Tumor cells are positive for cytokeratins (CAM5.2, CK19, AE1), RCC antigen, CA9 (Fig. 5.10d), CD10, EMA, napsin A [24], and vimentin and less frequently or weakly and focally positive for AMACR [AU: see earlier query Already added above], CD117, and E-cadherin [25]. Tumor cells are negative for CK7 and CK20.
For chromophobe RCC, the differential diagnosis includes oncocytoma/oncocytic carcinoma, clear cell RCC, and benign proximal renal tubular cells [22]. Tumor cells are positive for pan-cytokeratins, including CK7 (Fig. 5.11d), EMA, and CD117, and less frequently positive for RCC antigen, napsin A [24], E-cadherin, and CD10. The tumor cells are negative for vimentin, AMACR, and CK20 [25]. There is diffuse cytoplasmic Hale colloidal iron staining [37].
For papillary RCC, the differential diagnosis includes papillary adenoma (≤1 cm), well-differentiated papillary transitional cell carcinoma, clear cell RCC [27], and carcinoma of the collecting ducts of Bellini. Tumor cells are positive for cytokeratins (CAM5.2, AE1/AE3, and CK7), RCC antigen, CD10, EMA, AMACR (Fig. 5.12c), napsin A [24], vimentin, and S-100; they are less frequently positive for CD117 and CA9 (Fig. 5.12d) [40]. The type 1 tumor cells are negative for E-cadherin, and type 2 tumor cells are less frequently positive for E-cadherin [25].
If FNA only shows sarcomatoid features, differential diagnosis includes leiomyosarcoma, angiomyolipoma, and spindle squamous cell carcinoma or urothelial carcinoma. The tumor cells are positive for both keratins and vimentin.
For carcinoma of the collecting ducts of Bellini, tumor cells are positive for high molecular weight cytokeratins (34βE12, CK19), vimentin, CD15, EMA, and possibly mucin. Tumor cells are negative for CD10 and CD117 [25, 41].
Urothelial Carcinoma
Clinical
Radiology
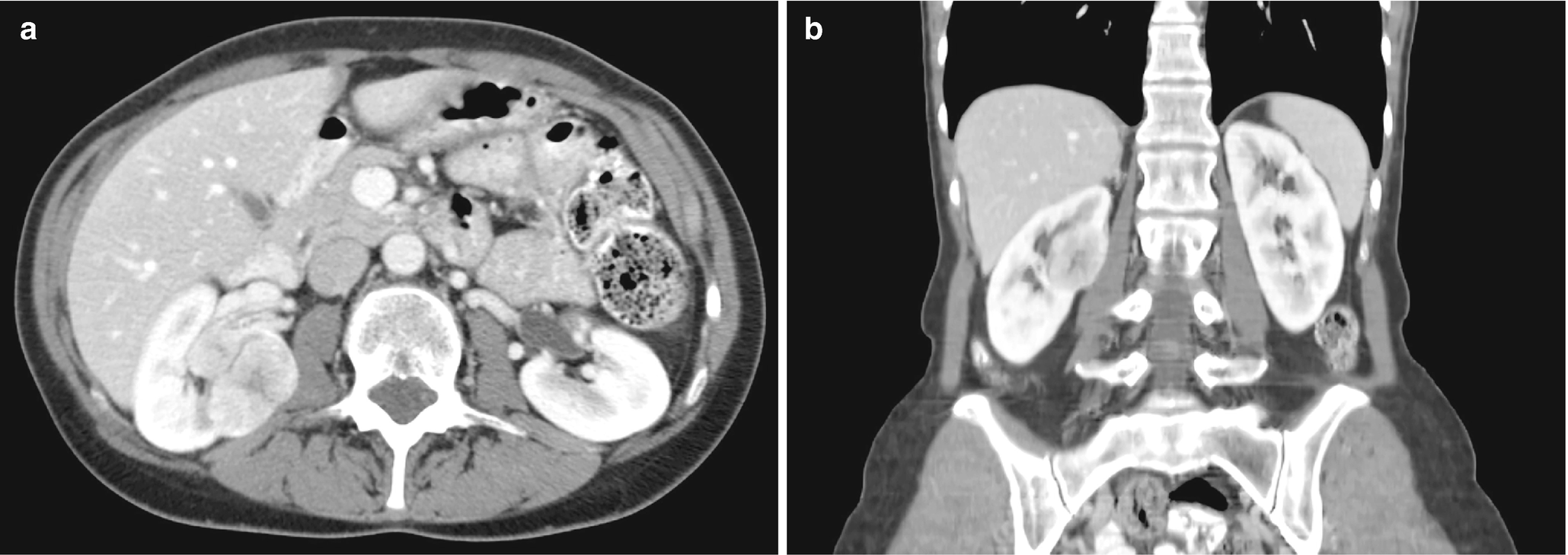
CT imaging features of urothelial carcinoma show possible thickening of the urothelial lining or a pelvi-calyceal filling defect (Fig. 5.14).
Occasionally, these may present as masses much like RCC; however, location in the collecting system, slight enhancement, and the absence of necrotic or cystic change increase the likelihood that the lesion is more likely to be urothelial carcinoma [43].
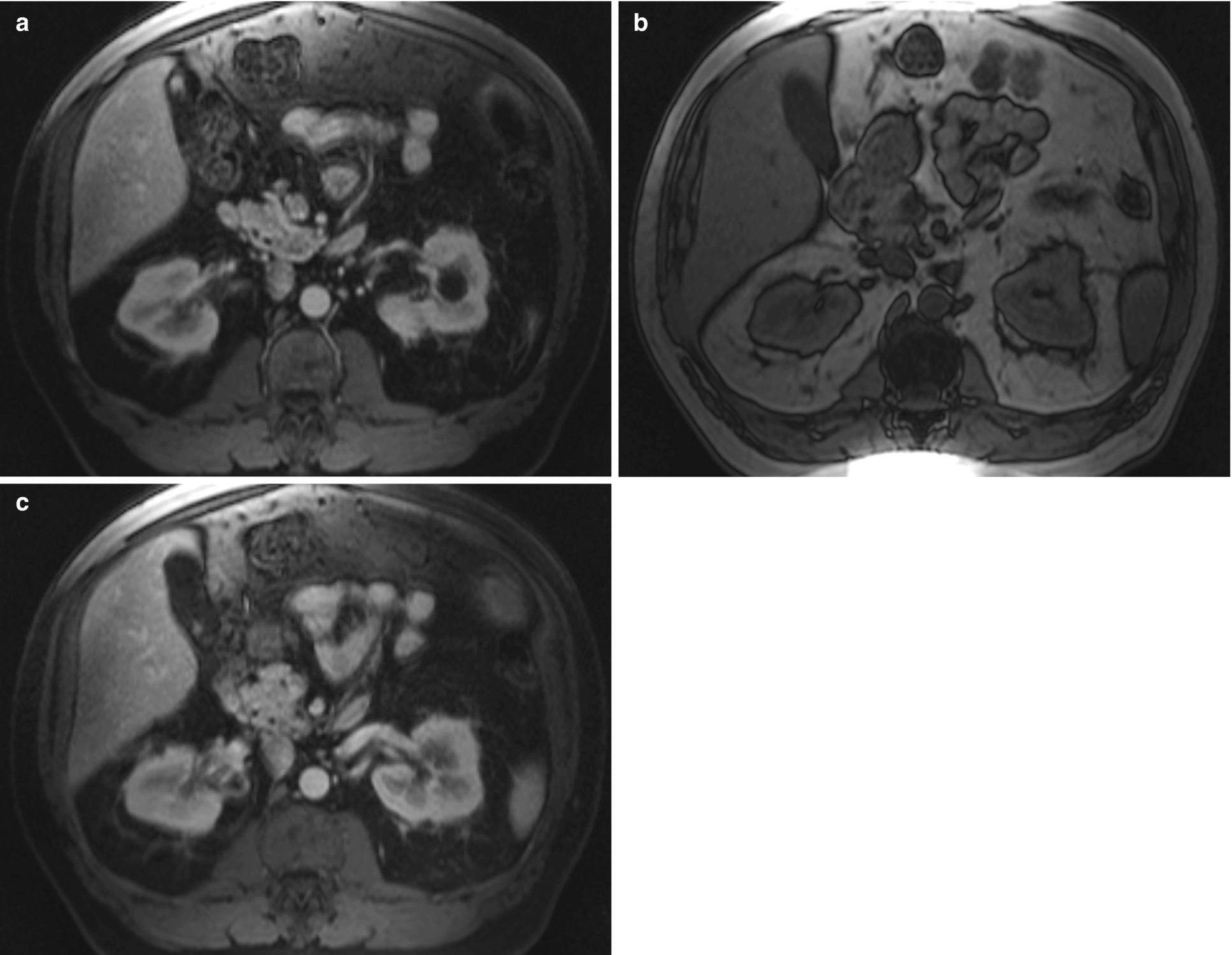
Urothelial carcinoma, radioimages . (a) CT scan shows a large infiltrating mass occupying the upper half of the left kidney involving the central collecting system. Few pathologic lymph nodes are seen in the adjacent region. (b) Another CT scan view of the large left renal mass which enhances after contrast administration. (c) Early excretory phase of CT scan again shows an extensive, infiltrative mass
Cytology of FNA and Touch Preparation of Core
Low-grade urothelial carcinoma: characteristically shows papillary aggregates of minimally atypical urothelial cells.
High-grade urothelial carcinoma: characteristically shows single or papillary aggregates of obvious malignant urothelial cells (Fig. 5.15). The nuclei are markedly pleomorphic and have coarse, irregular chromatin and prominent nucleoli. The cytoplasm is scant to moderate, glassy to dense, and occasionally vacuolated [6].
Micropapillary architecture might be seen in urine cytology [44] and biopsy.
Squamous differentiation, glandular cells, clear cells, or bizarre cells may be seen.
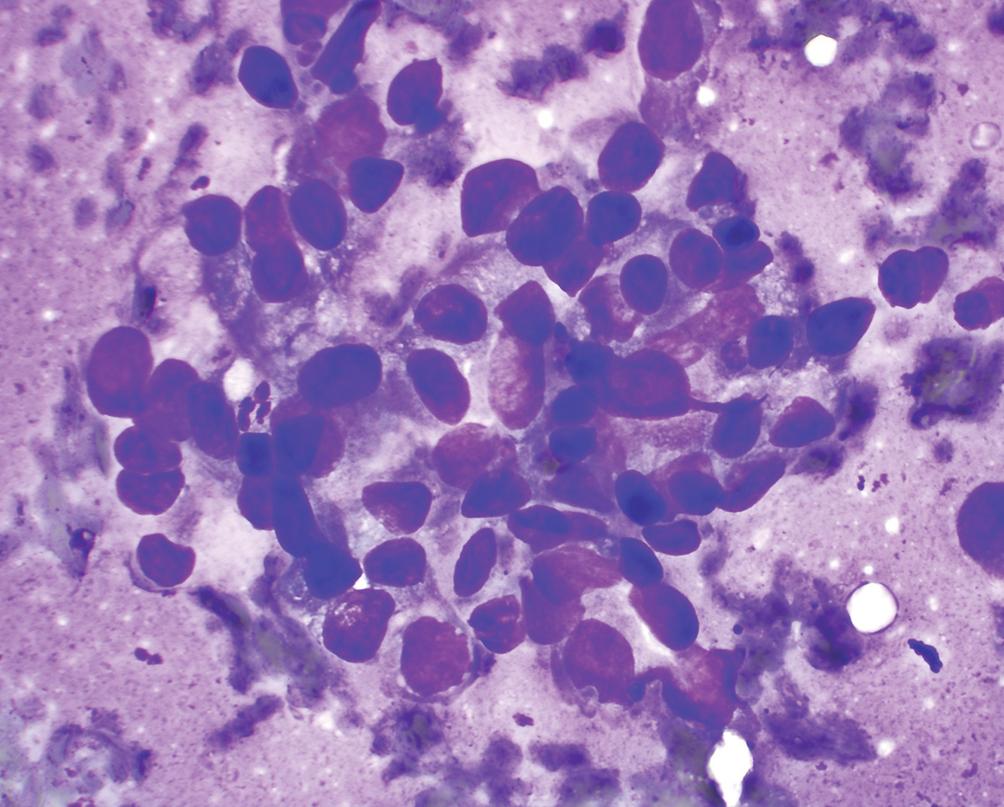
Urothelial carcinoma, high grade. FNA biopsy of a renal mass from a patient with an enlarged retroperitoneal lymph node; Diff-Quik stain, 600×
Comments
Differential diagnosis includes reactive urothelial cells caused by stones, inflammation, and instrumentation, metastatic carcinoma, sarcoma, high grade RCC, and papillary RCC.
Urothelial cells are positive for CK7, p63, p40, GATA3, CK20, CK5/6, K903, and thrombomodulin. GATA3 is more sensitive than traditional markers for conventional urothelial carcinoma and micropapillary urothelial carcinoma [45]. High-grade urothelial carcinoma tends to lose expression of p63 and p40, while it retains GATA3 expression [45]. Tumor cells are negative for napsin A [24].
Nephroblastoma (Wilms Tumor)
Nephroblastoma is a malignant embryonal neoplasm derived from nephrogenic blastemal cells that both replicates the histology of developing kidneys and often shows divergent patterns of differentiation [46].