Class of drug
Effect on urine/plasma normetanephrine and metanephrine
Tricyclic antidepressants
Increase
Tricyclic antidepressants (acute administration)
Decrease
Selective serotonin reuptake inhibitors
Increase
a-Adrenergic receptor blockers (selective)
No effect
a-Adrenergic receptor blockers (nonspecific)
Increase
β-Adrenergic receptor blockers
Increase
Monoamine oxidase inhibitors (MAOI)
Large increase
Adrenocortical Carcinoma
Adrenocortical carcinoma is a rare type of tumor that should be suspected when lesions are >6 cm in size and/or if the patient presents with virilizing symptoms. Patients with AI and possible ACC should be biochemically screened for hypersecretion. One study determined 66 % of ACC were hormone-secreting tumors [7, 8].
Diagnostic Evaluation
Imaging (CT, PET/CT, MRI)
It is important to choose the appropriately indicated study type to most accurately determine pathology. While the majority of AIs are benign, there are certain radiologic characteristics that are pathognomonic for specific lesions. Some AI can be immediately characterized with CT if certain features are clearly present. Cortical adenomas are homogenous and have low attenuation; myelolipomas usually display macroscopic fat; ACCs are usually large (>6 cm) and heterogeneous; and cysts show fluid density. There are also characteristics that may indicate malignancy such as local invasion of surrounding tissue, tumor necrosis, heterogeneity of mass, irregular borders, and regional lymphadenopathy. Any relevant prior imaging studies performed should be evaluated. If a lesion is stable for at least 6 months, the likelihood that the lesion is malignant is remote.
Most patients with AI will have undergone contrast-enhanced CT as the initial study but contrast-enhanced CT singularly has little use in characterizing adrenal lesion by densitometry. AI lesions can be characterized by an adrenal CT protocol including unenhanced CT followed by contrast washout measurements. On unenhanced CT, adrenal cortical adenomas normally have low attenuation (<10 HU), indicating a lipid-rich lesion, while ACCs more commonly have higher attenuation (≥10 HU), indicating a lipid-poor lesion. An increased probability of malignancy can be determined when contrast washout levels are evaluated. Most malignant lesions have an absolute washout of <60 % and a relative percentage washout of <40 %, while benign lesions show an absolute washout of >60 % and a relative washout >40 % [7, 9]. Absolute and relative washout are related to the characteristics of the lesion, with benign adenomas displaying a more rapid washout of contrast, while the opposite is true for malignant lesions. Each can be calculated as follows:
 The accuracy of MRI in distinguishing benign and malignant adrenal masses is similar to that of CT imaging. On MRI, normal adrenal gland tissue and adenoma demonstrate a low to intermediate signal on T1- and T2-weighted imaging, while malignant lesions are usually hypointense on T1 and hyperintense on T2 imaging. Adrenocortical carcinoma appears heterogeneous such as in CT imaging but may have a loss of signal intensity on out of phase MRI images leading to a mistaken diagnosis of benign lesion. The typical teaching that pheochromocytomas are usually hyperintense on T2-weighted images but may also exhibit low SI on T2-weighted images.
The accuracy of MRI in distinguishing benign and malignant adrenal masses is similar to that of CT imaging. On MRI, normal adrenal gland tissue and adenoma demonstrate a low to intermediate signal on T1- and T2-weighted imaging, while malignant lesions are usually hypointense on T1 and hyperintense on T2 imaging. Adrenocortical carcinoma appears heterogeneous such as in CT imaging but may have a loss of signal intensity on out of phase MRI images leading to a mistaken diagnosis of benign lesion. The typical teaching that pheochromocytomas are usually hyperintense on T2-weighted images but may also exhibit low SI on T2-weighted images.

Another useful tool for distinguishing benign from malignant lesions is 18-fluoro-2-deoxy-d-glucose position emission tomography, 18F-FDG-PET. Patients with a persistently indeterminate adrenal mass even following an appropriately performed CT should undergo an FDG-PET. Adrenal FDG uptake is considered malignant when intensity is higher than hepatic uptake [10]. Malignant tumors also have demonstrated larger max uptake of FDG when compared to benign lesions [11]. A large multicenter prospective study including 77 patients demonstrated that FDG-PET successfully distinguished ACC from adenoma with a sensitivity of 100 % and a specificity of 88 % [11]. However FDG-PET is primarily useful when attempting to diagnose the highly malignant ACC from an indeterminate nonfunction adenoma because FDG-PET may result in a false positive when imaging functional adenomas and pheochromocytomas since these lesions exhibit uptake similar to that of malignant lesions.
Adrenal Vein Sampling (AVS)
If a preliminary diagnosis of primary hyperaldosteronism is made, further biochemical testing in the form of adrenal venous sampling should be performed to confirm diagnosis. Adrenal venous sampling involves sampling blood from the adrenal veins and comparing the aldosterone concentration to the contralateral side and that found in the periphery. This technique will be discussed in-depth later in this chapter.
Indications and Contradictions for Adrenal Biopsy
Lesions definitively characterized as adenomas by imaging do not require biopsy. Functioning adenomas should be surgically excised while nonfunctioning adenomas should be followed up with interval imaging. Other benign nonfunctioning adrenal lesions include myelolipomas and adrenal cysts which also do not require biopsy. Suspected pheochromocytomas should not be biopsied; a thorough and properly performed biochemical assessment of plasma metanephrines can confirm this diagnosis. An unnecessary biopsy of a pheochromocytoma may cause a sudden hypertensive crisis due to the release of excessive catecholamines into circulation. These lesions should be treated with surgical resection. Lesions that cannot be confidently characterized by standard radiologic or laboratory criteria and lesions that are larger than 4 cm and lesions with markedly heterogeneous appearance on imaging should be referred for adrenal biopsy [12]. However, masses which are highly suspicious for adrenocortical carcinoma such as those isolated adrenal mass >4 cm with irregular margins/central necrosis and no known primary malignancy should be considered for wide surgical excision rather than biopsy. AIs found in patients with a medical history of extra-adrenal malignancy may be considered for adrenal biopsy. Mazziglia et al. showed that 40–75 % of AIs in this population indicate metastatic disease [13]. By comparison other studies have demonstrated a 0 % rate of malignancy in 973 patients with no history of cancer who presented with adrenal incidentaloma [14, 15]. Historically, percutaneous adrenal biopsy has had relatively limited indications due to high non-diagnostic rate, risk of tumor seeding, and possible catecholamine surge if the lesion may be a pheochromocytoma. A retrospective study performed by Mazzaglia et al. determined that needle biopsy of AI is often inadequate for distinguishing benign lesions from malignant lesions due to histologic similarity and recommend that biopsy only be considered for making diagnosis of metastatic disease in patients with known or suspected extra-adrenal malignancy [13]. As such, these lesions usually present as large (>4 cm), heterogeneous lesions.
Pre-procedure
Routine coagulation parameters such as INR and platelet count must be checked before biopsy to mitigate risk of hemorrhage. Anticoagulants should be discontinued at least 2–5 days prior to the procedure depending on the specific medication used (Table 27.1). In patients at high risk for deep vein thrombosis, a bridging technique can be utilized before and after biopsy using low-molecular-weight heparin [16]. Some radiologists will forego these recommendations with patients who are healthy and have no history of abnormal bleeding or use of anticoagulants. Adrenal biopsy is usually well tolerated and can be administered under local anesthesia or with conscious sedation. Prophylactic antibiotics are normally not recommended.
Relevant Anatomy and Procedure Technique
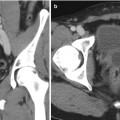
The adrenal glands are located in the retroperitoneum directly superior to the kidneys and encased in Gerota’s fascia (Fig. 27.1). There are rare anatomic variations in adrenal gland location. In the cases of solitary kidney or pelvic kidney, adrenal glands will be found in the same location but appear differently on imaging.
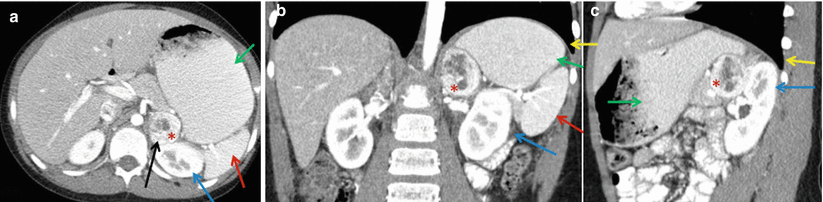

Fig. 27.1
Anatomical considerations for adrenal biopsy. Left-sided 4.3 cm adrenal mass (red asterisk). Paraspinous needle placement (black arrow) is often the safest approach given the close proximity of the spleen (red arrow), stomach (green arrow), kidney (blue arrow), and pleura (yellow arrow). Representative images of contrast-enhanced CT abdomen (a) axial view, (b) coronal view, and (c) sagittal view
In cases in which fine-needle aspirate is necessary, small-caliber needles (20–22 gauge) should be used. For histologic analysis, cutting needles are to be used to provide core biopsy samples. During adrenal biopsies, coaxial technique using 16–19 gauge guide needles is used to allow for the retrieval of fine-needle aspirates and core biopsies. Chiba needles with slightly curved tips are commonly used to enhance redirecting, while Hawkins needles with interchangeable tips can be utilized if saline injection is anticipated for paravertebral widening [17].
Patients are oriented in the decubitus position which decreases diaphragmatic movements, lessening the risk of lung transgression. Patients can also be placed prone in order to attempt a posterior approach to the adrenal glands. If neither approach works, an anterior approach can be used, though it is associated with higher rates of complications [18]. A caudal-to-cephalic approach may be utilized to minimize risk of pleural transgression in both the prone and decubitus positions. In cases where the paravertebral space is minimal, injection of saline can be administered to widen the space. The saline will displace the parietal pleura laterally, decreasing the risk of pleural transgression. Ultrasound guidance can be used for large adrenal masses and gives the advantage of real-time observation. Otherwise, CT guidance is the standard imaging modality used for adrenal biopsy.
Results and Complications
Complication rates for adrenal biopsy range from 0 to 12 % [19–22]. The most common complications associated with adrenal gland biopsy are pneumothorax and hemorrhage. Needle track seeding, hypertensive crisis, and adrenal abscesses are other less common complications that have be noted in literature. Following biopsy, patients usually remain under care for another 3 h to monitor hemostasis [17]. When post-biopsy hemorrhage does occur, it usually occurs in the abdomen or thorax and normally resolves without intervention [17]. Literature has demonstrated an increase in morbidity and mortality when the transhepatic approach and the anterior approach to the left adrenal gland which traverses the pancreas have been utilized [18]. In cases in which the pleural space was transgressed, a chest x-ray (CXR) must be ordered as well as 3 h follow-up CXR after the procedure to evaluate for a progressive pneumothorax. Patients found to have pneumothorax should be treated with a small-bore chest tube. Another serious complication of adrenal gland biopsy that may occur during the procedure is hypertensive crisis propagated by the release of catecholamines from the gland. Approximately 2.4 % of pheochromocytomas present atypically on imaging and thus can lead to unsuspecting biopsy of a catecholamine-secreting tumor [23]. If an acute hypertensive crisis does occur during biopsy, it can be treated with 1 mg of phentolamine via IV followed by an IV phentolamine drip. Intravenous nitroprusside can also be utilized to control hypertension as well as beta-adrenergic blockers to treat cardiac arrhythmias or hypotension. For lesions in which pheochromocytoma has not been conclusively ruled out, administration of alpha-blockers prior to the procedure should be considered.
The sensitivity of adrenal biopsy for detecting malignancy ranges from 81 to 100 % [24–26]. Several studies have reported that adrenal biopsy in this setting is unreliable and variable [13, 27, 28]. However, one prospective study of 220 adrenal biopsy demonstrated a correct diagnosis 76.8 % of biopsies. The study included 39 malignant tumors and showed a sensitivity of 94.6 % and a specificity of 95.3 % when determining malignancy [29]. While these results are exceptional, they may not be reflective of common adrenal biopsy practice since the radiologic selection criteria were very strict enriching the study cohort for malignant lesions; in addition, ACC-specific immunohistochemistry stains were used on core samples improving the diagnostic results as well. As it stands though, adrenal biopsy should be indicated for AI lesions that remain indeterminate after appropriately performed imaging (CT/PET and/or MRI) and biochemical assays which both may discourage adrenal biopsy.
Discussion
Adrenal gland lesions are extremely common and are often found incidentally on radiographic imaging studies performed for other reasons. The vast majority of lesions can be diagnosed accurately with radiographic criteria alone. Furthermore, the overwhelming prevalence of benign adenomas even in patients with a history of malignancy results in a limited role for adrenal biopsy. Most published data indicate that adrenal biopsies have an unacceptably high non-diagnostic rate and do not reliably differentiate between benign and malignant primary adrenal tumors. Additionally, a small but significant risk of a hypertensive crisis due to an unrecognized or atypical pheochromocytoma also limits the application of adrenal biopsy. Finally, the theoretical risk of tumor seeding and increasing complexity of subsequent surgery are additional concerns that limit widespread application of adrenal biopsy. Currently, the usage of core biopsy for the differentiation of benign tumors from malignant tumors in the adrenal gland is not part of standard diagnostic algorithms for adrenal masses.
Adrenal gland lesions are indicated for biopsy if the mass is indeterminate after imaging and the patient has a history of malignancy or if the mass is indeterminate and larger than 4 cm. All patients should undergo plasma screening for catecholamines prior to the biopsy to rule out pheochromocytoma. Adrenal biopsy is a generally well-tolerated procedure, and in experienced hands complication risks are low. In specific and well-characterized patient populations, biopsy can be useful for the diagnosis of adrenal incidentalomas.
Common Tips for Success
Target-side down decubitus positioning often is the safest paraspinal access.
To visualize a clear path for the biopsy needle, gantry angulation may be needed. Many CT scanners allow up to 30° of gantry tilt which is usually enough to visualize a clear access route.
Smaller Chiba and spinal FNA needles (21–23 ga) may be preferable if lesion appears hypervascular on pre-biopsy imaging.
Core biopsy specimens are preferred for diagnosis of lymphoma.
Etched needles may enhance visualization under ultrasound guidance, and manual scratching of needle with metal clamp will achieve effect with no added cost.
Adrenal Venous Sampling
Adrenal venous sampling is the current standard of care for identifying and lateralizing surgically curable cases of hyperaldosteronism caused by aldosterone-secreting adrenal tumors. Studies have reported sensitivity of 95 % and specificity of 100 % for adrenal venous sampling for lateralizing aldosterone hypersecretion [30–36]. This technique, though, remains underutilized. It is often described as a technically challenging procedure with problematical complications such as adrenal vein rupture and embolism [35, 37]. Adrenal venous sampling also lacks a universally accepted set of standards for performance and interpretation of results. The classic presentation of primary aldosteronism (PA) is hypertension with hypokalemia and metabolic alkalosis. Most times, though, patients do not present with all three symptoms. The Endocrine Society has established guidelines to screen select patient populations. Patients with hypertension and hypokalemia, refractory hypertension, hypertension with an adrenal incidentaloma, or hypertension with history of familial/early onset hypertension should be screened for primary aldosteronism [38]. Diagnostic workup for primary aldosteronism includes plasma renin assay (PRA) and plasma aldosterone concentration (PAC), both retrieved peripherally. Decreased plasma renin with an increased PAC/PRA ratio is indicative of primary aldosteronism. Normal PAC/PRA ratio is usually in the range of 4–10 while patients with PA will have a ratio of 30–50 [39, 40]. Recent studies have suggested that primary aldosteronism accounts for 5–13 % of all hypertensive patients and that up to 62.3 % of patients with primary hyperaldosteronism may be curable with surgical resection [40–42]. It is important to note that the two most common causes of primary hyperaldosteronism are bilateral idiopathic hyperplasia and unilateral aldosterone-producing adenoma (as well as a chance of bilateral functioning adenomas) [38, 43]. This distinction is critical because clinical management of patients depends on accurate diagnoses. Patients with bilateral hyperplasia must be managed medically due to the inability to treat surgically. These patients are best treated with long-term usage of spironolactone or other aldosterone antagonist [38]. Adrenal venous sampling can be used to accurately lateralize functioning adenomas for surgical resection. Recent studies have shown that localizing the source of aldosterone-producing adenomas and bilateral lesions using MRI and CT is difficult and often leads to equivocal findings [44]. One prospective study of 203 patients noted that up to 46.4 % of patients may have been incorrectly treated based on CT-imaging alone [36]. This includes patients that may have had an inappropriate adrenalectomy and patients that would have been excluded for adrenalectomy. Another large study showed 37.8 % of the 950 patient population had CT/MRI results in disagreement with adrenal venous sampling outcome [44]. Mounting evidence continues to demonstrate the need for AVS usage when CT imaging is equivocal and/or when lateralization of lesion(s) is required.
AVS Technique
Currently, there is no universally standard protocol for performing adrenal venous sampling. Adrenal venous sampling involves the catheterization of the left and right adrenal, sampling the venous drainage. There are several major variations in technique that can be employed when performing this procedure. Both adrenal veins can be sampled simultaneously using two catheters (Fig. 27.2) or sequentially using only one catheter at a time for each of the veins. Furthermore, the use of intraprocedural ACTH stimulation is another technical option dependent on practitioner preference. If ACTH stimulation is desired, cosyntropin, synthetic ATCH, can be administered as a bolus or as a continuous intravenous infusion. Sacks et al. suggest utilizing three initial sets of simultaneously obtained samples. This set is followed by administration of a 0.25 mg bolus of cosyntropin and removing samples at 5, 10, and 15 min intervals after bolus administration [45]. Mathur et al. utilized a different technique that included two baseline samples taken 5 min apart from both adrenal veins and the iliac vein [46]. After the initial samples, an intravenous bolus of 0.25 mg of ACTH followed by an infusion of ACTH of 0.25 mg in 250 mL of normal saline was given at a rate of 150 cc/h. Venous samples were collected at 5, 10, and 15 min intervals after ACTH infusion.
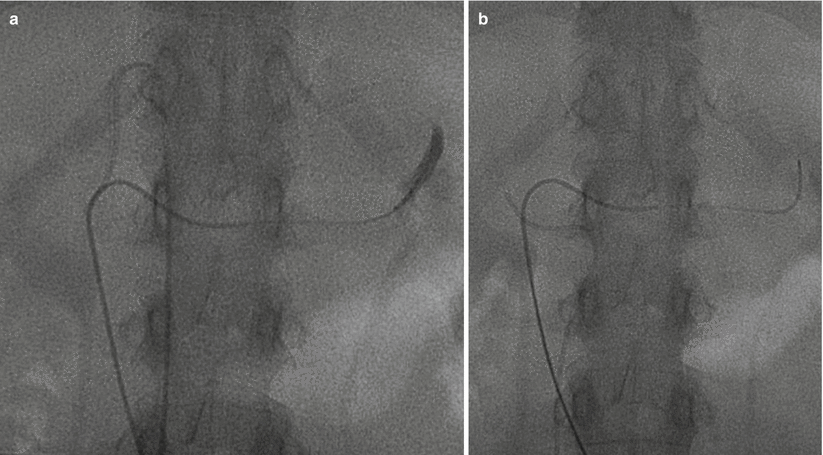

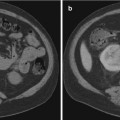
Fig. 27.2
Adrenal vein sampling. Two-catheter technique for AVS. Note the left catheter traversing the length of the left renal vein with cephalad deflection into the left adrenal vein. The right catheter is in the right adrenal vein and shorter as would be expected anatomically
The role of synthetic ACTH in AVS is to induce ACTH-sensitive adenomas to produce aldosterone and/or cortisol in excess of normal physiological levels. This process is the main mechanism behind AVS and allows clinicians to lateralize lesions to either or both adrenal glands. As stated before, there is no standard for whether a bolus, infusion, or both should be used during this procedure, though generally most centers use a combination of both techniques. There has been data published demonstrating the possibility that the administration of ACTH is unwarranted and may confound the results of the procedure [47]. One study investigating the usage of three different amounts of ACTH dosage (high, intermediate, and low) found that the high and intermediate dosage groups led to an incorrect lateralization in 3 and 12.5 % of cases, respectively [47]. The authors have suggested that this may be due to the tendency for the ACTH to stimulate the normal adrenal glandular tissue instead of the adenoma. As of now, more data is needed to determine the optimal protocol for administration of ACTH stimulation.
The Adrenal Venous Sampling International Study showed that about 66 % of centers used sequential sampling technique with cosyntropin stimulation [48]. The remaining centers used simultaneous sampling with no cosyntropin stimulation. Although there is the possibility of creating an artificial gradient when sampling sequentially, when cortisol secretion is maximally stimulated using cosyntropin, the time difference between sampling is irrelevant when assessing selectivity. The pulsatile nature of aldosterone secretion, though, makes measurements susceptible to temporal variability in aldosterone concentration in each adrenal vein. This fact is important to consider for determining the lateralization index (LI).
Once the catheter has been placed, samples are removed and assayed to determine what is referred to as the selectivity index (SI). This index is used to confirm the placement of the catheter by comparing the concentrations of cortisol from the adrenal vein samples to samples taken from the inferior vena cava. A selectivity index, defined as plasma cortisol concentrationadrenal vein/plasma cortisol concentrationIVC, ≥2–5 indicates cortisol levels are more concentrated in the adrenal veins and confirms selectivity [46, 48–50]. Another way to confirm placement of the catheter is using gentle retrograde venography. Contrast filling the right and left adrenal veins confirms placement. The simultaneous and sequential techniques are described below.
The simultaneous technique is started when catheters with side holes located toward the distal end (generally 4-F Simmons 1 and 4-F Simmons 2 catheters; Cook, Bloomington, IN) are directed through the right common femoral vein and placed in the right and left adrenal veins [51]. Small amounts of nonionic contrast are injected to verify the locations of the catheters. During this time, a 3000 unit bolus of intravenous heparin should be administered. The baseline samples are removed simultaneously from the right and left adrenal veins to be tested for aldosterone and cortisol concentrations. Cosyntropin is administered as a 0.25 mg bolus followed by 0.25 mg in 5 mL of normal saline infused over 10–15 min. After ACTH stimulation via cosyntropin, blood is removed simultaneously from the right and left adrenal veins. Following the completion of the infusion, blood is drawn from the IVC.
In the sequential technique, a single set of post-infusion samples are obtained following bilateral catheterization. Patients receive a continuous intravenous infusion of 0.25 mg per 500 mL of normal saline of cosyntropin at a rate of 100 mL/h. Infusion is initiated 1 h before sampling as well as throughout the procedure. Access to the right adrenal vein is gained through the right common femoral vein with a 5-F HS2 catheter (Cook) with a side hole located near the distal end [51]. A sample is drawn to determine aldosterone and cortisol concentrations. As stated above, retrograde injection is used to verify catheter location. Directly following this, the catheter is switched for a 5-F Simmons 2 catheter and catheterization and sampling of the left adrenal vein is performed. Once again, retrograde injection is performed to verify catheter location. Drawn blood samples are measured for aldosterone and cortisol concentrations. The catheter is then retracted into the IVC where another set of samples are drawn for aldosterone and cortisol measurement [51].
Diagnostic Evaluation
Once the PAC/PRA ratio is used to confirm a diagnosis of primary aldosteronism, PRA, PAC, and a basic metabolic panel may indicate the etiology of the PA. Unilateral aldosteronomas are responsible for 30–60 % of cases and usually cause greater hypertension, greater hypokalemia, and higher concentrations of aldosterone when compared to bilateral adrenal hyperplasia [46]. Literature has reported a sensitivity of 100 % and specificity of 61 % in diagnosing aldosteronomas using a PAC/PRA ratio >32 [37]. If findings remain equivocal, the lateralization index can be used to assess the location of aldosterone hypersecretion. The LI is defined as PAC/PCCdom: PAC/PCCnondom




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree