actual practice in the moving, crying infant. To help solve this problem, a variety of immobilization devices are available for children <4 years old. Pacifiers are also useful. An important caveat is that pediatric patients with suspected airway obstruction should never be forced into a position they do not wish to assume, because this may lead to acute respiratory decompensation, which is potentially life threatening.
TABLE 8.1 Radiography Technique for Soft Tissue Neck | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(representative parameters: 5,000/34, echo train length of 12, 22-cm field of view, 6-mm section thickness with 2-mm gap, 256 × 192 matrix, two signals acquired) sequences are obtained. Cine MR is performed in midline sagittal and axial planes at the midportion of the tongue using a fast gradient-echo sequence (representative parameters: 8,200/3,600, 80-degree flip angle, 12-mm section thickness) with 128 consecutive images captured in ˜2 minutes and viewed on cine mode.2,16
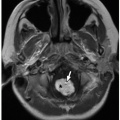
and soft palate, uvula, oropharynx, tongue, mandible, base of the tongue, vallecula, epiglottis, aryepiglottic folds, pyriform sinuses, laryngeal ventricle, true and false vocal cords, subglottic larynx, and upper trachea (Fig. 8.2).
TABLE 8.2 Tube Current and kV by Patient Weight for Central Airway MDCT | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
breathers.2 Associated anomalies such as coloboma, heart defects, and mental retardation and syndromes including CHARGE (coloboma, heart disease, choanal atresia, growth and mental retardation, genital hypoplasia, ear anomalies with deafness), Crouzon, Pfeiffer, Antley-Bixler, Marshall-Smith, Schinzel-Giedion, and Treacher Collins occur in ˜50% of affected patients.2,21,23
 FIGURE 8.12 Normal anatomy of the upper airway. T1-weighted sagittal MR image shows normal anatomy of the upper airway. |
nasopharyngeal and oropharyngeal obstruction. Potential sequelae include chronic hypoxemia and hypercarbia from hypoventilation and OSA.2,14,33,34,35,36,37
enlargement, particularly when associated with airway compromise or OSA, adenoidectomy and/or tonsillectomy may be required. Surgery may also help relieve associated recurrent sinus or ear infections.2
trapping, persistent cough, stridor, acute respiratory distress, or hemoptysis.63,64 A classic history is persistent right upper lobe atelectasis following endotracheal intubation because of obstruction of the unsuspected aberrant bronchus by the endotracheal tube.25,26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree