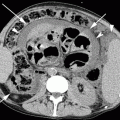
Fig. 14.1
(a, b) Chest X-ray (a) and unenhanced CT coronal MIP reconstruction (b) demonstrate diffuse calcifications of the tracheobronchial tree in an 82-year-old woman
2.
The trachea may be more displaced to the right side than usual, due to the enlargement of the atherosclerotic aortic arch which may simulate a mediastinal mass.
3.
“Saber-sheath” tracheal deformity is common in advanced age and almost exclusively occurs in men. It may be considered a pathognomonic sign of chronic obstructive pulmonary disease (COPD), characterized by an increase in lung volume that restricts the side-to-side dimensions of the paratracheal mediastinum, forcing the trachea to remold itself into a saber-sheath configuration (Greene 1978). The coronal diameter of the intrathoracic trachea is markedly reduced, whereas the sagittal diameter is increased with a sagittal-to-coronal diameter ratio exceeding 2:1. The extrathoracic trachea is normal in diameter. CXR shows diffuse narrowing of the intrathoracic trachea on the posteroanterior view (Fig. 14.2a). CT scans may depict an inward bowing of the lateral tracheal walls (Fig. 14.2b, c), due to cartilage weakness, which may be accentuated on the expiratory scans. In the early stage, tracheal narrowing may be appreciated only at the thoracic inlet.
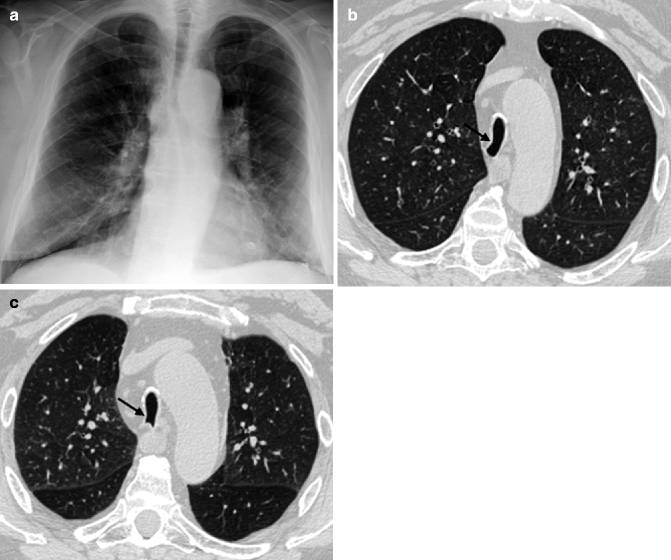

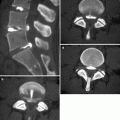
Fig. 14.2
(a–c) “Saber-sheath” trachea in a 73-year-old man with COPD. Chest X-ray posteroanterior view (a) shows a narrowing of the transverse diameter of the intrathoracic trachea. Axial inspiratory (b) and expiratory (c) CT images at lung window setting better depict the inward bowing of the lateral tracheal walls (arrows)
4.
Bronchial wall thickening due to collagen deposition and bronchial dilation may be part of the normal spectrum of the “senile lung.” This airway modification associated with an increase of supporting interstitial connective tissue becomes superimposed on the vascular network, giving rise to the definition of “dirty chest” on CXR (Bonomo et al. 2008; Copley et al. 2009).
Only a few studies in the literature specifically focus on the CT appearance of healthy lungs and even fewer on healthy elderly lungs, due to the obvious difficulties in obtaining lung biopsy confirmation of thin-section CT findings in apparently healthy individuals (Hansell 2010).
14.3 Classification
The airways are divided into upper and lower. Some diseases typically affect the upper tract and others the lower tract. Many disorders involve both, as there is a single airway.
Upper airway diseases represent a heterogeneous group of disorders. The majority of tracheobronchial diseases are uncommon and often remain undiagnosed and untreated. In the elderly, single entities may be masked by the presence of comorbidities and by the use of multiple therapies. Older patients are often malnourished and immunocompromised, more prone to develop severe complications. For these reasons, it is essential to center the diagnostic process on the individual. Signs and symptoms are often aspecific, but their clinical course may be informative. Recent improvements in imaging of the tracheobronchial tree have made physicians increasingly aware of this heterogeneous group of diseases.
Lower airway diseases generally involve the bronchioles as a predominant feature, but they can also involve more proximal airways or spread distally towards lung parenchyma. Airflow limitation, that may or may not be fully reversible, is the hallmark of these entities. Lower airway diseases represent one of the leading causes of morbidity and mortality among the elderly due to the decline of local and systemic defenses, poor nutritional status, and comorbidities (Comino et al. 2006). They usually remain undetected on conventional radiography and functional tests until there is widespread involvement. High-resolution CT has advanced our understanding of small airway diseases and has greatly enhanced diagnostic accuracy in the evaluation of patients suspected of having small airways diseases, in particular when correlated with clinical features.
14.4 Imaging Modalities
Chest radiography usually represents the first step in the evaluation of airway diseases. Nevertheless, it is usually aspecific in this clinical context, and often the involvement of airways is unapparent or missed at CXR and diagnosis is often delayed.
Multidetector computed tomography (MDCT) is currently the best imaging technique for the assessment of the airway diseases, from the trachea to the most distal visible airways. Due to its high temporal and spatial resolution, MDCT allows the assessment of the entire thorax in around 5–6 s while using 1 mm or less slice thickness. Thus, slice misregistration and respiratory motion artifacts, often relevant in the elderly, are significantly reduced. An additional expiratory CT scan may be requested to evaluate the degree of tracheobronchomalacia and the extent of air trapping, a sign of small airway disease (Beigelman-Aubry et al. 2009). Low kilovoltage (100–120 kV) and milliamperage (60–160 mAs) may be used without significant loss of information because of the unique natural contrast between the airways and their environment.
MDCT generates isotropic voxels and allows high-quality two-dimensional multiplanar (MPR) and tridimensional reformations, as volume rendering (VR). MPR images are oriented in any plane, allowing a better evaluation of the extent of the lesion and the identification of subtle stenoses. MPR images may also be combined with the use of intensity projection techniques: maximum intensity projection (MIP) and minimum intensity projection (minIP). MIP images clearly depict the regional distribution of inflammatory centrilobular nodules, facilitating the recognition of small or poorly defined centrilobular nodules and tree-in-bud opacities. Conversely, minIP images enhance visibility of the airway within the lung parenchyma and the assessment of central airway stenoses and improve the detection of regional heterogeneity of lung parenchyma attenuation (mosaic pattern) and of air-trapping extensions (Beigelman-Aubry et al. 2009).
VR techniques provide an accurate map of the central airways (via CT bronchography) and a simulation of endoscopic navigation through the tracheobronchial tree (via virtual bronchoscopy) The results look more familiar to surgeons and bronchoscopists than axial images (Thomas et al. 2009). Virtual bronchoscopy is a useful, noninvasive alternative to the conventional method in patients with severe stenoses, beyond which a conventional bronchoscope cannot pass, and in certain subgroups of patients, such as older and very sick patients, who may not be able to tolerate invasive exams. However, conventional bronchoscopy remains primary in the diagnostic work-up of patients with abnormalities of the central airways, allowing tissue sampling and definitive diagnosis (Marchiori et al. 2008).
Recent advances in MDCT technology and software implementation permit automated detection and quantification of airway disease, in particular of small airway disease and pulmonary emphysema in COPD patients. Recognition of the predominant COPD phenotype (emphysema predominant, airway-disease predominant, mixed type) may improve treatment with targeted therapy for each patient.
14.5 Upper Airway Diseases in the Elderly
A variety of diseases may affect the upper airways, either nonneoplastic or neoplastic. The former are usually classified into focal and diffuse, depending on the extension of the lesion; the latter are distinguished as benign or malignant. In Table 14.1, all the possible causes of upper airway disease in the elderly are listed.
Table 14.1
Causes of upper airway disease in the elderly
Nonneoplastic disease | Focal | Narrowing airway lumen | Secretions |
Broncholithiasis | |||
Stenosis (iatrogenic and postinfectious) | |||
Tuberculosis | |||
Diffuse | Enlarging airway lumen | Tracheobronchomegaly | |
Tracheobronchial diverticula | |||
Narrowing airway lumen | Tracheobronchomalacia | ||
Amyloidosis | |||
Tracheobronchopathia osteochondroplastica | |||
Bronchial anthracofibrosis | |||
Wegener granulomatosis | |||
Sarcoidosis | |||
Relapsing polychondritis | |||
Neoplastic disease | Benign | Surface epithelium: | |
Solitary squamous cell papilloma | |||
Salivary glands: | |||
Pleomorphic adenoma | |||
Mesenchyme: | |||
Hamartoma | |||
Lipoma | |||
Chondroma | |||
Schwannoma | |||
Leiomyoma | |||
Granular cell tumors | |||
Malignant | Primary | Surface epithelium: | |
Squamous cell carcinoma | |||
Adenocarcinoma | |||
Large cell undifferentiated carcinoma | |||
Neuroendocrine tumors: | |||
Small cell lung cancer | |||
Carcinoids (more atypical) | |||
Large cell neuroendocrine carcinoma | |||
Salivary glands: | |||
Adenoid cystic carcinoma | |||
Mucoepidermoid carcinoma | |||
Carcinoma ex pleomorphic adenoma | |||
Mesenchyme: | |||
Lymphomas | |||
Sarcomas | |||
Chondrosarcoma | |||
Secondary | Direct invasion by adjacent malignancy | ||
Hematogenous metastases |
Among the nonneoplastic causes, focal abnormalities usually affect a single region of the airways and cause a narrowing of the lumen. The most common in the elderly include endoluminal secretions, broncholithiasis, tracheal stenoses (iatrogenic and postinfectious), and tuberculosis. Diffuse diseases involve a long segment of the airway or have multiple foci and may cause airway enlargement or narrowing. The most common in the elderly are tracheobronchomegaly, tracheobronchial diverticula, and tracheobronchomalacia, but we would also include less usual diseases that radiologists should recognize, such as tracheobronchopathia osteochondroplastica, amyloidosis, Wegener granulomatosis, and bronchial anthracofibrosis. Some of these entities may have a focal or diffuse appearance, such as tuberculosis, postinfectious stenoses, amyloidosis, and Wegener granulomatosis. Among the neoplastic causes, primary tracheobronchial neoplasms are usually rare and malignant in the elderly (80–90 %).
14.5.1 Nonneoplastic Tracheobronchial Disease
14.5.1.1 Focal Nonneoplastic Diseases
Secretions
Mucus is the most common abnormality of the tracheobronchial tree seen on CT scans in the elderly, and in particular in patients with chronic bronchitis and in bedridden patients. It usually presents low attenuation and a “bubbly” appearance, owing to the commixture with air. Secretions commonly occur along dependent portions of the airway, but it also may be adherent to the nondependent walls, making differential diagnosis with a tumor difficult. In these cases, repeating the CT scan after a vigorous cough may be helpful (Marom et al. 2001).
Broncholithiasis
Broncholithiasis is defined as a condition in which calcified or ossified material (broncholith) is present within the lumen of the tracheobronchial tree. There are three main causes: (1) erosion by and extrusion of calcified peribronchial lymph nodes (Fig. 14.3a), (2) aspiration of radiopaque fragments or in situ calcification of foreign material, and (3) erosion by and extrusion of calcified bronchial cartilage plates (Seo et al. 2002).
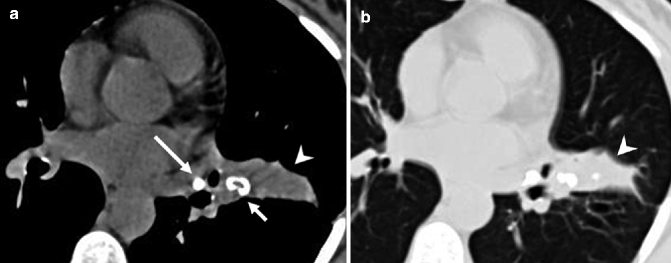

Fig. 14.3
(a, b) Broncholith in a 67-year-old woman. Axial non-enhanced CT image at mediastinal window setting (a) shows a calcified nodule, broncholith (short arrow), that proximally occludes the lumen of a subsegmental bronchus of the lingula and determines distal postobstructive parenchymal atelectasis (arrowhead), also shown at lung window setting image (arrowhead) (b). Note a calcified peribronchial lymph node in the left hilum (long arrow)
Erosion by calcified peribronchial lymph nodes is usually associated with long-standing foci of necrotizing granulomatous lymphadenitis, frequent in tuberculosis and histoplasmosis. Preferential sites are the proximal right middle lobe bronchus and the origin of the anterior segmental bronchus of the upper lobes. In addition to typical CT findings of broncholithiasis, lymph nodes in other sites and parenchymal abnormalities due to infection appear.
The elderly are more prone than younger adults to foreign body aspiration due to the increased frequency of neurologic dysfunction, loss of consciousness, facial trauma, intubation, dental procedures, and the use of sedatives. Foreign bodies may already be calcified or calcium may deposit around them. Radiologic findings include radiopaque nodules within the tracheobronchial tree with or without postobstructive changes.
The common calcifications of tracheobronchial cartilage in advanced age may produce a subsequent sequestration of the calcified material into the bronchial lumen, generating broncholiths (Seo et al. 2002).
Broncholithiasis is often associated with postobstructive abnormalities, including bronchiectasis, obstructive consolidation (Fig. 14.3a, b), and air trapping (Grenier et al. 2009a). Owing to frequent coagulation disorders and immunodeficient status of the elderly, complications like massive hemoptysis and obstructive pneumonia are common. Thus, broncholithiasis produces significant morbidity in the elderly and its management may be difficult. In recent years endobronchial therapies with YAG laser (yttrium aluminum garnet) have been introduced. Accurate preprocedural planning with MDCT imaging and good quality multiplanar reconstructions enhance the bronchoscopist’s knowledge of the relationships between the target lesion and critical structures and improve the efficiency of specific endobronchial therapies (Ferguson et al. 2006).
Stenoses
Acquired tracheobronchial stenoses are more commonly iatrogenic and postinfectious.
Iatrogenic stenoses are usually a complication of tracheal intubation or tracheostomy, or occasionally may be secondary to lung transplantation. The placement of endoluminal tubes in the large airways may cause a pressure necrosis of the tracheal mucosa, impeding local blood circulation (Grenier et al. 2009a). Subsequently, the combination of necrosis and reparative fibrosis leads to a progressive stenosis, usually in the subglottic region, at the cuff site or at the stoma site. Dyspnea on exertion, stridor, and wheezing are the most common symptoms.
Iatrogenic stenoses are usually focal, 1.5–2.5 cm in length, showing a concentric soft-tissue thickening of the internal mucosa, while the outer tracheal wall has a normal appearance (Webb et al. 2000). Eccentric tracheal narrowing may also occur (Fig. 14.4a). CXR often does not reveal the tracheal stenosis, as it usually lies on the upper edge of the radiograph (Prince et al. 2002). On CT longitudinal images, the focal narrowing may produce a typical “hourglass” configuration (Grenier et al. 2009a) (Fig. 14.4b).
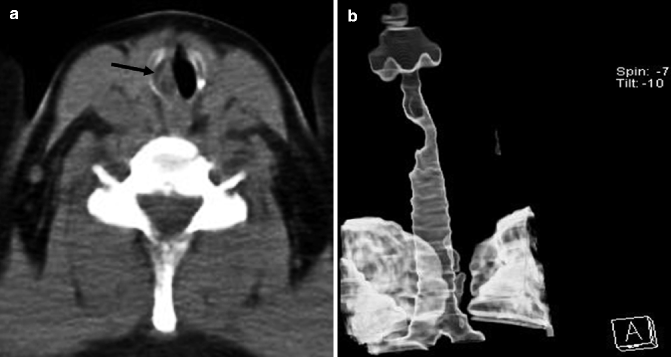

Fig. 14.4
(a, b) Post-intubation tracheal stenosis in a 65-year-old man. Axial non-enhanced CT image at mediastinal window setting (a) shows an eccentric stenosis of the subglottic tracheal lumen on the right (arrow). External VR image (b) along the long axis of the trachea better depicts the stricture extent and the typical “hourglass” appearance of the trachea
Stenosis after lung transplantation usually appears as a focal narrowing at the site of bronchial anastomosis at CT and bronchoscopy (Laroia et al. 2010).
Postinfectious strictures are mainly caused by Mycobacterium tuberculosis and may occur at the same time as infection (owing to inflammatory edema) or also 30 years later (for fibrotic modifications) (Prince et al. 2002). Other pathogens which may cause central airway narrowing include Histoplasma capsulatum, Aspergillus species, Klebsiella rhinoscleromatis, and Coccidioides immitis (Marom et al. 2001). Histoplasmosis is the most common endemic mycosis causing hospitalization in the United States, and the severity of illness depends on the intensity of exposure (to birds and bat droppings) and the immunity of the host (Youness et al. 2009). Elderly people are at high risk for disseminated disease because of their weak immunity.
Imaging of postinfectious stenoses demonstrates narrowing with or without calcifications of the trachea or bronchi and segmental or lobar atelectasis. CT depicts the focal or diffuse tracheobronchial wall thickening with luminal strictures better than CXR (Prince et al. 2002). Nevertheless, axial images may overlook subtle stenosis and underestimate its craniocaudal extent (Boiselle et al. 2008). Nowadays, MDCT with 2D and 3D reconstructions is the exam of choice for the identification and evaluation of the site and the length of stenosis and residual airway lumen (Laroia et al. 2010). MPR and VR reconstructions are essential for treatment planning and subsequent follow-up.
Tuberculosis
The geriatric population, among all ethnic groups and both genders, represents the largest reservoir of tuberculosis (TB) infection, particularly in developed nations. The elderly are at a greater risk for reactivation of latent TB and for the acquisition of new TB infection. Underlying acute or chronic diseases, malnutrition, and biological changes occurring with aging can impair microbial clearance mechanisms and contribute to the expected age-associated decline in cellular immune response to infective agents such as Mycobacterium tuberculosis (Rajagopalan and Yoshikawa 2000). The high number of cases diagnosed at autopsy among the elderly suggests that this condition often remains unrecognized, possibly due to the subtle clinical manifestations in this age group (Zevallos and Justman 2003).
Central airways may be involved in 10–40 % of cases of pulmonary parenchymal tuberculosis, by lymphatic spread or local invasion from mediastinal affected lymph nodes (Prince et al. 2002). Pathogenesis begins with the formation of tubercles in the submucosa, which subsequently lead to ulceration and necrosis. Finally, the healing process leads to a variable degree of fibrosis, narrowing the lumen. The fibrotic phase of disease is resistant to medical therapy; thus, a stent placement is often necessary to recover bronchial patency.
MDCT findings tend to reflect the stage of disease, differentiating the active phase, which is characterized by an irregular thickening of tracheal wall (due to inflammatory edema), from the fibrotic phase, in which the tracheal narrowing shows a smooth and normally thick wall. However, up to 20 % of bronchial stenoses are caused by extrinsic lymph node compression or infiltration. The strictures are more commonly focal than diffuse, concentric, and usually 3 cm in length. The most frequent site of stenosis is the lower third of the trachea and the main stem bronchi (Fig. 14.5a–d).
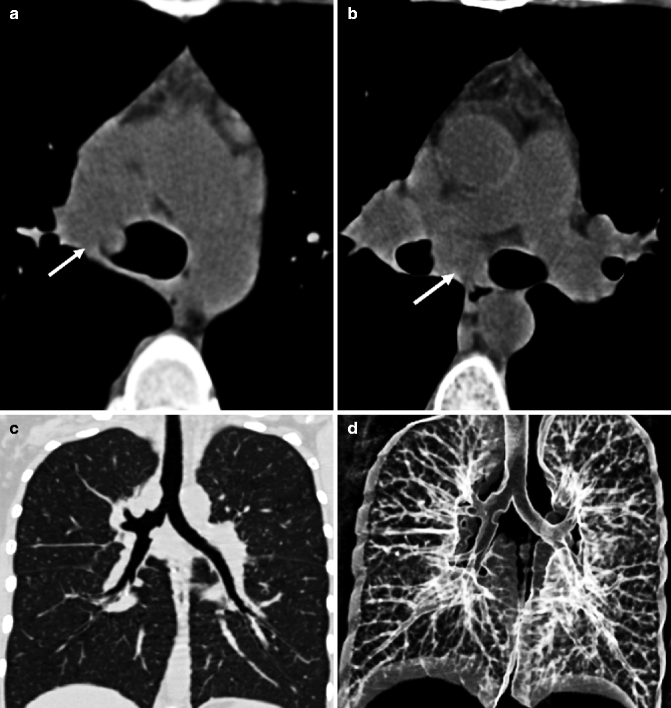

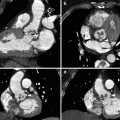
Fig. 14.5
(a–d) Tracheobronchial tuberculosis. Axial non-enhanced CT images at mediastinal window setting (a, b) show enlarged lymph nodes (arrows) in the right paratracheal and subcarinal regions that infiltrate distal trachea and right main bronchus walls and determine airway lumen narrowing. Coronal MPR image at lung window setting (c) better depicts the sites of stenoses, at the right tracheobronchial corner and at the right main stem bronchus, with smooth lobulated margins. External VR image (d) shows the locations of tracheobronchial strictures
Differential diagnosis mainly includes bronchogenic carcinoma, which usually involves a shorter airway segment and presents as an intraluminal mass with eccentric luminal narrowing. Endobronchial biopsy is necessary, though it has proven to be nonspecific for tuberculosis in one-third of the cases (Grenier et al. 2009a).
14.5.1.2 Diffuse Nonneoplastic Diseases
Tracheobronchomegaly
Tracheobronchomegaly is a rare condition characterized by marked dilation of the trachea and main stem bronchi, due to a severe atrophy of longitudinal elastic fibers and thinning of the muscularis mucosa (Laroia et al. 2010). It occurs when the transverse and sagittal diameters of the trachea exceed 25 and 27 mm, respectively, or the left and right main stem bronchi diameters exceed 18 or 21 mm, respectively, in men. In women, the respective diameters are 21, 23, 17.4, and 19.8 mm (Marom et al. 2001). Tracheobronchomegaly may be congenital or acquired. Congenital disorders associated with tracheobronchomegaly are Mounier-Kuhn, Marfan, and Ehlers-Danlos syndromes (Ahn et al. 2011). Acquired disorders include pulmonary fibrosis of the upper lobes (Fig. 14.6a, b) or chronic airway inflammation/infection (smoking, chronic bronchitis, emphysema, cystic fibrosis, relapsing polychondritis, allergic bronchopulmonary aspergillosis, radiotherapy) (Woodring et al. 1989; Marom et al. 2001). This disease primarily affects men in their fourth and fifth decades, but in literature, cases have been reported from 18 months to 79 years. Usually patients are asymptomatic or complain of signs and symptoms related to recurrent infections and bronchiectasis, as the disease progresses. Tracheobronchomalacia, bronchiectasis, and multiple tracheobronchial diverticula are commonly associated with this disease.
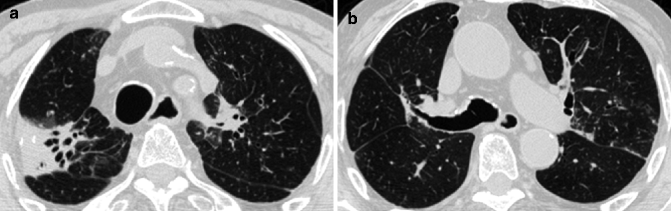

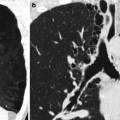
Fig. 14.6
(a, b) Tracheobronchomegaly in a 75-year-old man with pulmonary fibrosis of the upper lobes. Axial CT images at lung window setting show severe dilation of the trachea (a) and the right main stem bronchus (b) associated with fibrotic consolidations and traction bronchiectasis in the upper lobes, more evident on the right (a)
CXR shows dilation of the trachea and main bronchi, sometimes accompanied by an irregular profile of the airways, due to prolapsed redundant mucosa through the tracheal rings. These findings are better depicted by CT, which may also reveal tracheobronchial diverticula (multiple and with a broad base), central bronchiectasis, and the possible causes of tracheobronchomegaly, such as emphysema or fibrosis of the upper lobes. Expiratory CT scans may also reveal associated tracheobronchomalacia and air trapping.
Tracheal and Main Bronchial Diverticula
Tracheobronchial diverticula are more frequent among patients aged 50–83; therefore, the typical patient is elderly (Goo et al. 1999). These entities are asymptomatic and rarely diagnosed in most cases. They represent an incidental finding in chest CT scans, occurring in approximately 1–2 % of cases (Polverosi et al. 2008). Tracheobronchial diverticula may be congenital or acquired, with different pathogenesis, location, and imaging features.
Congenital diverticula are found predominantly in men, present the same anatomical structures as the tracheal wall (mucosa and submucosa layers and cartilage), and tend to arise on the right tracheal wall, 4–5 cm below the vocal cords and above the carina (Soto-Hurtado et al. 2006). They are usually very small, with a very narrow communication with the airway and typically filled with mucus. Congenital diverticula are not usually detected in infancy, unless they act as a reservoir for secretions causing recurrent tracheobronchial infections or unless they are associated with other congenital anomalies, such as tracheoesophageal fistulas.
Acquired diverticula are made up of only the mucosa layer. They most commonly arise along the posterolateral tracheal wall at the cervicothoracic junction, but they may arise at any level of the trachea and bronchi. They are large and usually have a wide communication with the airway (Polverosi et al. 2008). Their development seems to be related to an increase in intraluminal pressure, followed by the herniation of mucosa through a locus minoris resistentiae of the bronchial wall. Nevertheless, it has been demonstrated (Polverosi et al. 2008) that acquired diverticula occur not only in diseases characterized by chronic cough (i.e., COPD) but also in asymptomatic patients, underlining the great influence of tracheal wall anatomical characteristics rather than chronic stress in the pathogenesis of diverticula.
On CT scans, diagnosis of air paratracheal cysts first necessitates determining whether their origin is tracheal or not. Differential diagnosis includes Zenker diverticulum, pharyngocele, laryngocele, apical lung hernia, and bullae due to paraseptal emphysema. Diagnostic exams helpful in distinguishing between these disorders are a barium study of the digestive tract (for Zenker diverticulum and pharyngocele) and MDCT that allows visualization and the characterization of the lesion (location, size, number, origin, diverticula neck size, association with other pulmonary findings such as COPD). A lung window setting may be helpful in determining the neck of the diverticula, though it may not be visible if small (Fig. 14.7a, b). Expiratory scans highlight the air cyst changes in size if connected to the airways. MPR and VR reconstructed images better depict the relationship between the tracheal lumen and the air cyst in most cases. The use of contrast material is not helpful because the lesion lacks enhancement. MDCT may also identify rare complications (such as wall inflammation, perforation, and pneumomediastinum).
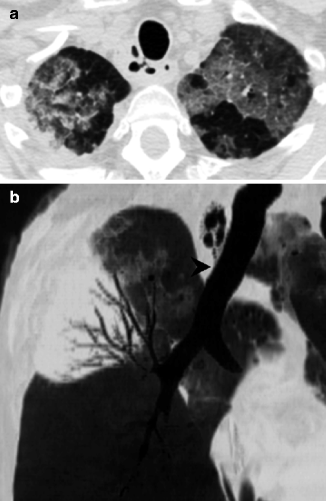

Fig. 14.7
(a, b) Incidental finding of tracheal diverticula in a 66-year-old immunocompromised patient with recurrent pulmonary infections. Axial CT image at lung window setting (a) shows air-filled right paratracheal cysts in apparent communication with the tracheal lumen by a narrow neck. Parasagittal minIP reformation (b) may help to confirm the presence of a small neck (arrowhead). Note the ground-glass opacities in the apical regions of both lungs and consolidation with air bronchogram in the right upper lobe, due to Legionella pneumonia (a, b)
In symptomatic patients, sometimes fiber-optic bronchoscopy may be used for pretreatment planning. Treatment options depend on the patient’s age, state, and symptoms. Due to the lack of severe symptoms and frailty of elderly patients, surgical treatment of tracheobronchial diverticula is uncommon and reserved for young patients.
Tracheobronchomalacia
Tracheobronchomalacia is not uncommon, although the true incidence is unknown because it is frequently underestimated. Tracheomalacia (TM) refers to a higher trachea susceptibility to collapse, due to reduction and/or atrophy of longitudinal elastic fibers of the pars membranacea of the trachea or to impaired cartilage integrity. TM may be localized to one portion of the trachea, or may involve the entire trachea, extending sometimes into the main stem bronchi, giving rise to tracheobronchomalacia (TBM). Rarely, the involvement of only one or both main stem bronchi may occur (bronchomalacia). Weakness of the tracheal and bronchial wall accentuates the physiologic changes of airway diameters (Carden et al. 2005).
TM may be congenital or acquired. Usually congenital forms are diagnosed in children, whereas the acquired forms affect mostly the middle-aged and the elderly (Carden et al. 2005). Risk factors include tracheostomy, tracheal intubation, thoracic surgery, radiation therapy, chest trauma, chronic inflammation (recurrent infections), irritants (cigarette smoke, gas), chronic ab estrinseco compression of the trachea (thyroid goiter, abscesses, neoplasms, cysts, aortic aneurysms, vascular rings, skeletal abnormalities), and relapsing polychondritis. Elderly patients with TBM usually complain of dyspnea and wheezing. Pulmonary function tests are not specific, as the obstructive profile is similar to patients with emphysema without TBM (Carden et al. 2005).
Typically, in patients with TBM there is either complete (easy to assess by visual analysis) or incomplete collapse of the airway. MDCT is considered the best imaging tool for accurate qualitative and quantitative analyses of the tracheobronchial lumen modifications.
As TBM escapes detection on standard end-inspiratory MDCT scans, diagnosis usually requires airway assessment after a forced expiration (static expiratory scan) or during an active respiratory maneuver (such as forced exhalation or coughing). As dynamic expiratory CT scans elicit a higher level of airway collapse than static end-expiratory scans in patients with TBM, the dynamic expiratory protocol may be the preferred method of imaging in these patients (Baroni et al. 2005). In a recent study (Lee et al. 2007), MDCT has been proved to have a similar sensitivity (97 %) to the traditional gold standard – bronchoscopy – for diagnosing TBM, and it represents a valuable alternative to bronchoscopy in the elderly population. In particular, in very elderly patients and in those with hearing impairments, MDCT with coughing maneuver should be preferred, because it can be difficult for the elderly to perform effective forced expiratory scans (Lee et al. 2007; Ridge et al. 2011).
A hallmark of TM is the “frown sign,” a crescent bowing of the posterior membranous tracheal wall at expiratory CT scans (Boiselle and Ernst 2006) (Fig. 14.8a–d). The quantitative method for assessing airways consists of electronic tracing of a cross-sectional area of the airway lumen at the same level in inspiratory and expiratory scans. A reduction of 50 % in cross-sectional luminal areas has been considered the diagnostic threshold for a long time, but recent studies recommend a cutoff of narrowing greater than or equal to 70–80 % on forced expiration, as even healthy subjects may exceed the current diagnostic criterion (50 %) (Boiselle et al. 2009; Carden et al. 2005; Grenier et al. 2009b; Heussel et al. 2001; Stern et al. 1993). Currently, a comprehensive diagnostic criterion is still lacking, so further studies are needed to elucidate the normal range of tracheal collapsibility and the pathologic cutoff.
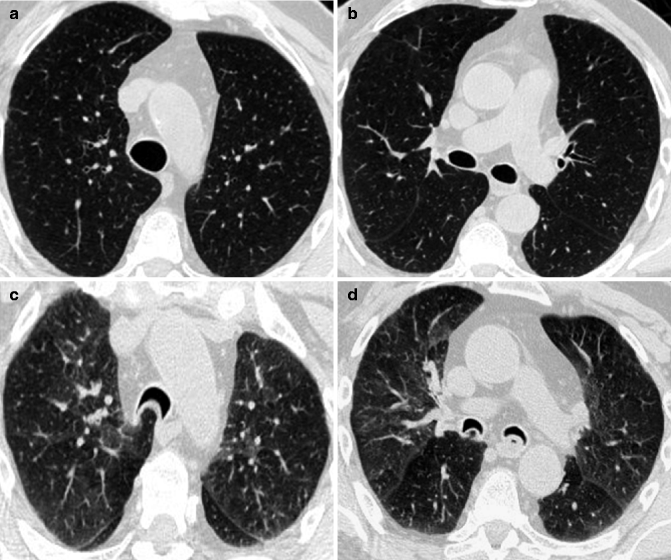

Fig. 14.8
(a–d) Tracheobronchomalacia in a 70-year-old man with COPD. Axial inspiratory CT images at lung window setting (a, b) show a normal shape of the tracheal and bronchial lumen. Expiratory CT images at lung window setting (c, d) demonstrate a severe collapse of the airways with an inward bowing of the posterior wall (“frown” sign), consistent with tracheobronchomalacia. Mild air trapping is also evident in both lungs (c, d)
To choose the best treatment, the severity, distribution, and morphology of airway collapse should be assessed (Lee et al. 2009). Severity may be evaluated by reporting a quantitative degree of airway collapsibility and by classifying it as mild (50–74 %), moderate (75–99 %), or severe (100 %) (Gilkeson et al. 2001). Distribution may be multifocal (two contiguous regions or at least two noncontiguous regions involved) or diffuse (more than two contiguous regions involved). Morphology may be distinguished as circumferential collapse, as excessive posterior wall bulging or as anterolateral cartilaginous aspect collapse.
Airway malacia has been associated with several comorbidities, most notably COPD. The precise mechanisms that explain this strong relationship are still unknown. It has been suggested that the loss of parenchymal elastic fibers may determine the instability of the tracheal wall, or mechanical stress due to chronic cough may cause stretching and degeneration of the posterior membranous portion of central airways (Sverzellati et al. 2009). Nevertheless, TBM is not related to the obstruction severity in COPD (Loring et al. 2007; Baram and Smaldone 2006). As recently demonstrated, airway malacia defined as ≥50 % may occur in roughly half of the patients with COPD across all the GOLD (Global Initiative for Chronic Obstructive Lung Disease) stages (Sverzellati et al. 2009).
Tracheobronchopathia Osteochondroplastica
Tracheobronchopathia osteochondroplastica (TO) is a rare, benign disorder of the submucosal tissue of the trachea and main stem bronchi. This disorder occurs most frequently in men over 50 years old, although a wide age range has been reported (25–85 years old) (Ferretti et al. 2009; Jabbardarjani et al. 2008; Pinto et al. 2010). Only half the patients are diagnosed in their lifetime, as many patients may remain asymptomatic and diagnosis is often incidental during a bronchoscopic exam. The most common symptoms, caused by a superimposed infection or endobronchial obstruction, include chronic cough on exertion, sputum production, hemoptysis, and dyspnea. Congenital anomalies, chronic irritation (cigarette smoking), or infection and metabolic disorders are believed to play an important role in the metaplastic changes of the tracheal elastic tissue.
CXR is normal in many cases, but irregularities and thickening of the trachea or a lobar collapse may be seen (Tadjeddein et al. 2006). MDCT is an important imaging modality in the diagnosis of TO, as it may reveal, in almost all cases, numerous submucosal sessile cartilaginous or bony nodules (3–8 mm), protruding into the tracheal lumen from the anterolateral walls, sparing the membranous posterior wall (a typical sign of TO) (Prince et al. 2002; Grillo 2004). TO more frequently involves the trachea and main stem bronchi (52 %) or the lower tract of the trachea (42 %), whereas the main bronchi are less frequently a single site of TO (6 %) (Pinto et al. 2010).
The “gold standard” for diagnosis of TO is bronchoscopy, showing glossy sessile cartilaginous and/or bony nodules with normal overlying mucosa, which spare the posterior tracheal wall. These nodules are typical and pathognomonic of TO.
Differential diagnosis (in older patients) mainly includes tuberculoid calcifications, amyloidosis, and Wegener granulomatosis (Pinto et al. 2010).
Tracheobronchial Amyloidosis
Amyloidosis is a rare disease caused by abnormal extracellular deposition of amyloid. This disorder may be hereditary or acquired, systemic or localized in single organs.
The respiratory system may be involved either in systemic or in localized forms. In systemic amyloidosis the respiratory tract is involved in 30–90 % of patients, usually in diffuse distribution (Dahl et al. 2004). The localized form of the respiratory tract is very rare and may be divided into three groups: tracheobronchial, nodular parenchymal, and diffuse parenchymal. The first, tracheobronchial amyloidosis (TBA), is the most frequent form and usually demonstrates diffuse involvement of the tracheobronchial tree, beginning from the trachea and large bronchi, extending sometimes to segmental bronchi. Rarely, the amyloid deposition is focal (amyloidomas), thus mimicking an endobronchial tumor (Kwong et al. 1992). TBA usually manifests in the fifth or sixth decade of life, but can range from 16 to 85 years, mostly in men (Gibbaoui et al. 2004). TBA is characterized by diffuse submucosal plaques and nodules of amyloid, which may infiltrate all layers of the airway wall, leading to erosion of cartilage and causing diffuse airway stenosis. Symptoms of TBA may vary according to the anatomic distribution of amyloid deposition: dyspnea, cough, and wheezing in the case of proximal tracheal disease or recurrent airway and parenchymal infections in distal tracheal and main bronchial disease (Gibbaoui et al. 2004).
In half of the patients, CXR is normal, whereas the remaining cases manifest lobar atelectasis, bronchial calcifications, bronchiectasis, and hilar adenopathy (Santos et al. 2008). MDCT clearly depicts a multifocal, nodular, or circumferential wall thickening of soft-tissue density with a smooth surface and possible concentric calcifications even in the posterior wall membrane, cartilaginous ossifications in absence of malacia, and postobstructive pulmonary complications (Kirchner et al. 1998; Webb et al. 2000) (Fig. 14.9a–d). Mural calcifications are prominent features that have to be distinguished from calcified lymph nodes (Grenier et al. 2009a).
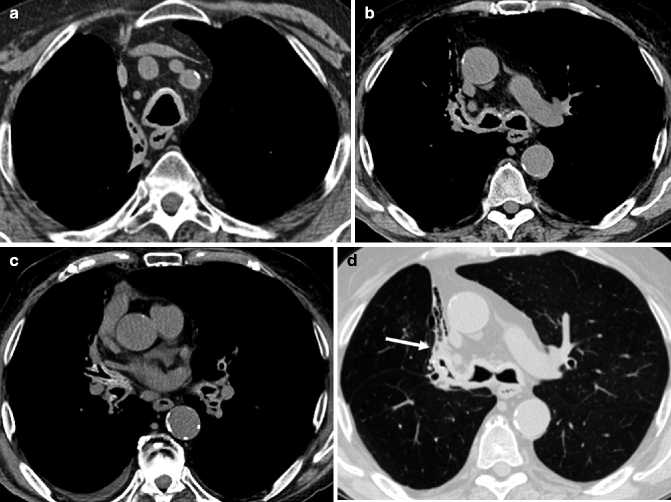

Fig. 14.9
(a–d) Tracheobronchial amyloidosis in a 77-year-old man with increasing dyspnea. Axial non-enhanced CT images at mediastinal window setting (a–c) demonstrate diffuse, circumferential, and smooth wall thickening with mild calcifications of the trachea, main stem bronchi, and lobar bronchi bilaterally. Axial image at lung window setting (d) shows an almost complete occlusion of the right upper lobar bronchus with a distal chronic postobstructive atelectasis of the anterior segment of the right upper lobe (arrow)
Although MDCT findings in severe TBA are highly suggestive of the disease, fiber-optic bronchoscopy allows the visualization of widely dispersed lesions with different colors and size and the collection of tissue samples necessary for definitive diagnosis (Gibbaoui et al. 2004; Santos et al. 2008).
Differential diagnosis includes firstly tracheobronchopathia osteochondroplastica (which typically spares the posterior wall membrane) and relapsing polychondritis, then granulomatous disorders (Wegener granulomatosis and sarcoidosis), severe chronic tracheobronchitis (like those associated with colitis ulcerosa), and neoplastic diseases (lymphoproliferative disorders, primary or secondary neoplasms).
Wegener Granulomatosis
Wegener granulomatosis (WG) is a multisystemic disorder of unknown etiology, characterized by a necrotizing granulomatous vasculitis which primarily affects the respiratory tract, lungs, and kidneys. Tracheobronchial involvement occurs in up to 16 % of patients, often as a late complication of the disease (Ingegnoli et al. 2007). In a series of 30 patients, Lee et al. (2003) reported large airway involvement in 30 % of patients (9/30) and segmental and subsegmental bronchial involvement in 73 % of patients (22/30). Rarely is the trachea the only site of involvement (Screaton et al. 1998).
WG occurs more frequently in middle-aged men, but in literature a wide age range is reported (20–83 years). Dyspnea, stridor, wheezing, cough, and hemoptysis are the most common symptoms (Daum et al. 1995).
CXR rarely depicts tracheal stenoses, which are better assessed by MDCT. A focal or, more often, diffuse circumferential wall thickening, concentric or eccentric, approximately 5–45 mm in length (mean, 25 mm), may be shown. Mural thickening may narrow the axial luminal area as much as 100 %, but usually it is restricted to 47 % (Screaton et al. 1998). Involvement of the cartilaginous rings is unusual, but it may lead to calcifications, erosion, and airway deformation. When evaluating tracheal strictures due to WG, the larynx should be looked at, as it most commonly affects the subglottic area. MDCT also allows the evaluation of lung parenchyma, frequently affected by the disease with evidence of parenchymal nodules (often cavitated), airspace consolidations, pleural effusion, or lobar-segmental atelectasis (Ingegnoli et al. 2007; Polychronopoulos et al. 2007).
Bronchoscopic manifestations include tracheobronchial stenosis with or without inflammation and ulcerating tracheobronchitis. Unfortunately, in an already compromised airway, bronchoscopic instrumentation and biopsy can lead to hemorrhage or edema, resulting in a further narrowing of the airway. Moreover, biopsy specimens from the upper respiratory tract have a notoriously low yield (Prince et al. 2002; Screaton et al. 1998). Determining c-ANCA (antineutrophil cytoplasmic antibodies) levels in the serum may help in the diagnosis of WG as changes in c-ANCA usually correlate with overall disease activity. Nevertheless, they do not reflect the tracheobronchial activity in most patients, and they should not affect management decisions regarding airway involvement (Daum et al. 1995).
Tracheal stenosis is often unresponsive to systemic therapy and local intervention is favored. MDCT, with MPR and VR images, is essential in pretreatment evaluation of tracheobronchial WG, by demonstrating optimal sites of tracheostomy and the true extent of the disease, when high-grade stenoses preclude complete bronchoscopic evaluation (Grenier et al. 2009a).
MDCT is also valuable in the follow-up of patients who have had endobronchial therapies or surgical interventions.
Bronchial Anthracofibrosis
Bronchial anthracofibrosis is a benign condition only recently defined. The term refers to the bronchoscopic finding of dark anthracotic pigments in the bronchial wall, with subsequent bronchial narrowing or obliteration, in patients without a relevant history of pneumoconiosis or smoking (Kang 2011). Bronchial anthracofibrosis occurs more commonly in elderly women, who complain of chronic cough, sputum, and dyspnea (Park et al. 2008; Ahn et al. 2011). The pathogenesis remains unknown. It has been suggested that lymph nodes with anthracotic material may perforate into the adjacent bronchi and the black pigments penetrate into the bronchial wall, coloring the mucosa layer. A fibrotic response may occur, resulting in bronchial narrowing with anthracotic pigmentation (Chung et al. 1998).
Chung et al. (1998) and Long et al. (2005) have suggested a strong relationship between tuberculosis and bronchial anthracofibrosis. Conversely, Park et al. (2008) reported that endobronchial tuberculosis seems not to be a major causative factor in anthracofibrosis, as coexistence of active tuberculosis occurs in only 20 % of patients. Furthermore, Park et al. underlined the role of MDCT in differentiating endobronchial tuberculosis from anthracofibrosis. The former usually shows a single lobar bronchial stenosis extending into the ipsilateral main bronchus and the trachea, whereas the latter presents multifocal stenoses in segmental and lobar bronchi of both lungs, sparing the main bronchi and trachea.
The most commonly reported finding on CT scans is lobar or segmental atelectasis distal to the involved bronchi (Grenier et al. 2009a). Multifocal stenoses of lobar and segmental bronchi with peribronchial soft-tissue thickening and calcified or noncalcified lymph nodes may also be seen (Ahn et al. 2011). Right upper lobe and right middle lobe bronchi are the most commonly involved (Kang 2011; Park et al. 2008). Differential diagnosis, beyond endobronchial tuberculosis, includes malignancies.
14.5.2 Neoplastic Tracheobronchial Diseases
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree