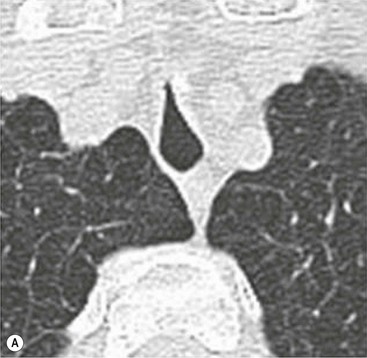
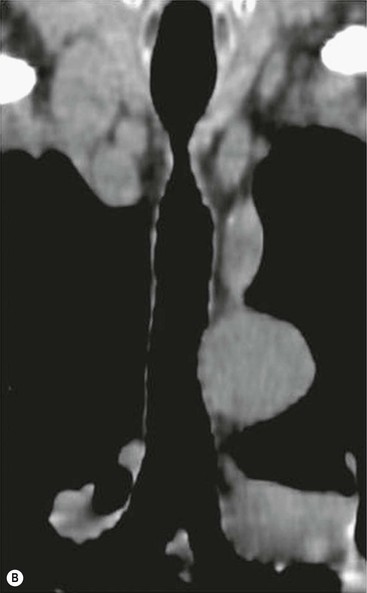
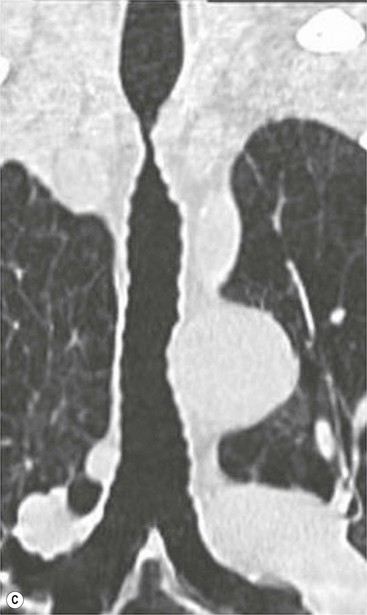
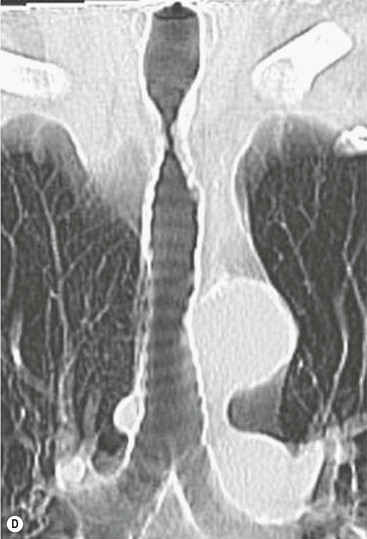
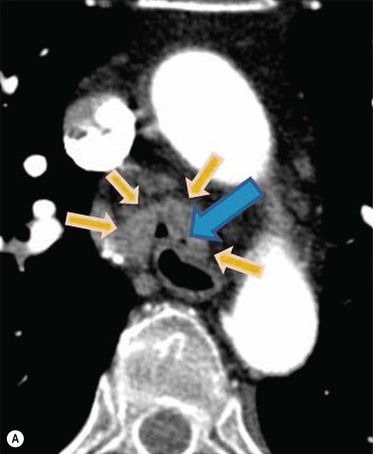
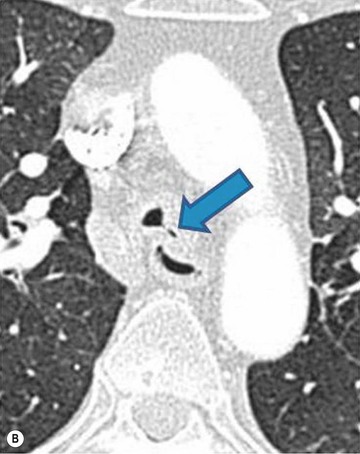
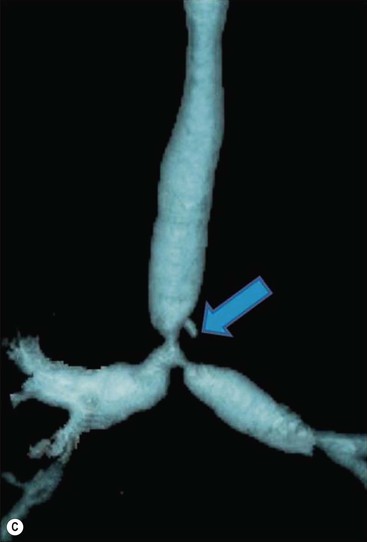
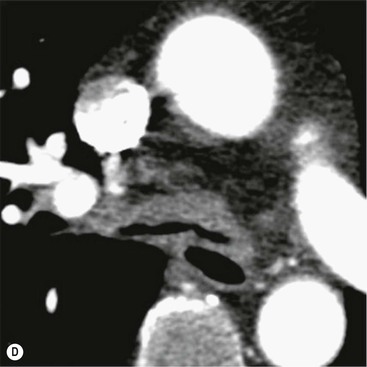
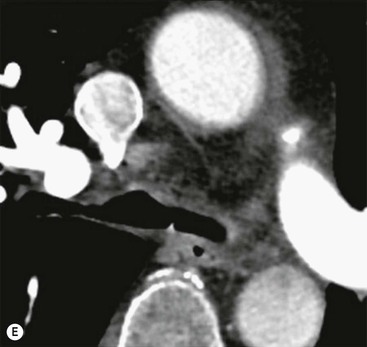
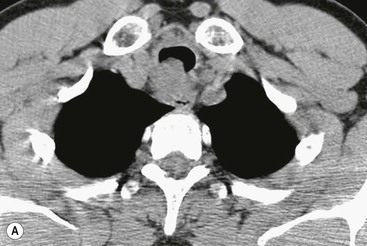
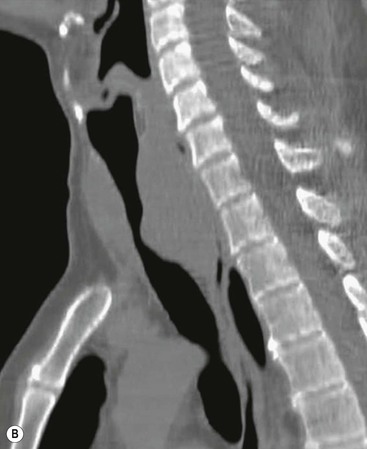
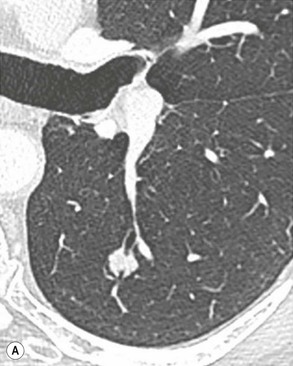
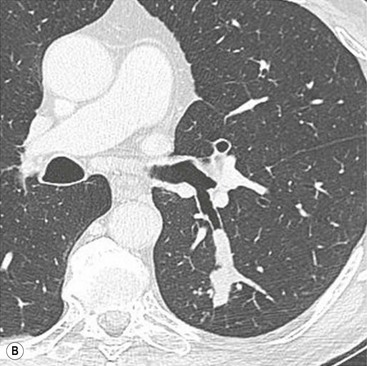
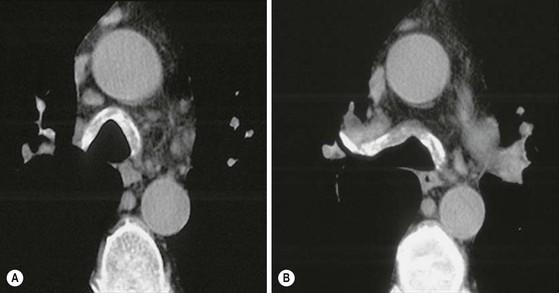
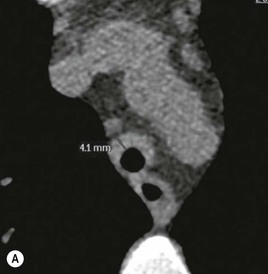
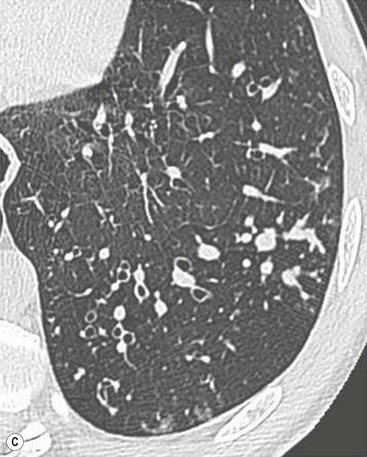
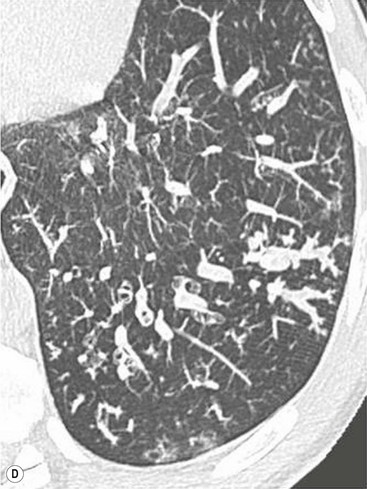
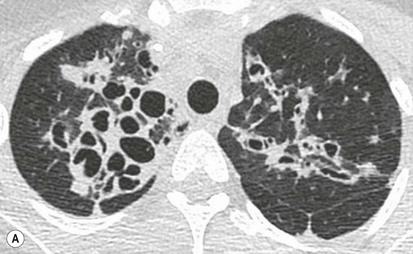
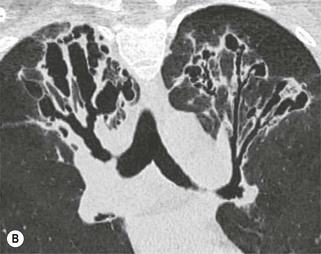
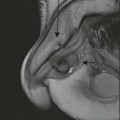
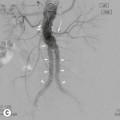
Philippe A. Grenier, Catherine Beigelman-Aubry The purpose of this chapter is to review lesions involving the trachea and proximal bronchi, to describe the radiological signs of bronchiectasis and to discuss the role of imaging in obstructive lung disease, a group of diffuse lung disease associated with chronic airflow obstruction that includes chronic obstructive pulmonary disease (COPD), asthma and obliterative bronchiolitis. In obstructive lung disease, decreased expiratory flow may be related to loss of lung recoil or small airway obstruction or combination of both. The abnormality which is better correlated with loss of recoil is emphysema. The process that causes the small airway obstruction is inflammatory in nature and is characterised by thickening of all the layers of the bronchiolar walls as well as an accumulation of mucus in the airway lumen (COPD and asthma), and/or an irreversible fibrosis (COPD and obliterative bronchiolitis). The trachea may be affected by a variety of extrinsic or intrinsic processes. Extrinsic processes, particularly masses, displace and distort the trachea, while intrinsic ones cause narrowing, widening or a mass effect. Tracheal narrowing may affect a short or a long segment, and may extend to the mainstem bronchi. Tracheal disease, often initially missed on CXR, is usually evident on careful evaluation of the frontal and lateral radiographs. CT allows precise delineation of the intratracheal and extratracheal extent of the abnormality. Multidetector CT, by combining helical volumetric CT acquisition and thin collimation during a single breath-hold, provides an accurate assessment of proximal airways, allowing multiplanar reformations and 3D rendering of high quality. Complementary CT acquisition at suspended or continuous expiration allows for assessing tracheal collapsibility. Strictures of the trachea are usually secondary to damage from a cuffed endotracheal or tracheostomy tube or to external neck trauma. The lesions consist of granulation tissue leading to dense mucosal and submucosal fibrosis associated with distortion of cartilage plates. The two principal sites of stenosis following intubation or tracheostomy are at the level of the stoma or the endotracheal balloon. On radiography, the stenosis may be seen as a focus of circumferential or eccentric narrowing associated with a segment of increased soft tissue. The size of narrowing is usually well seen at CT and is often concentric. Post-intubation stenosis extends for several centimetres and is typically seen above the level of the thoracic inlet. Post-tracheostomy stenosis typically begins 1–1.5 cm distal to the inferior stromal margin and extends for 1.5–2.5 cm. Multiplanar reformations accurately determine the site, the length and the degree of the stenosis (Fig. 13-1). In selected cases, the degree of stenosis may also be attained by use of virtual bronchoscopy. A number of infections, both acute and more often chronic, may affect the trachea and proximal bronchi, resulting in both focal and diffuse airway disease. Subsequent fibrosis may result in localised airway narrowing. The commonest causes of infectious tracheobronchitis are bacterial tracheitis in immunocompromised patients (Fig. 13-2), tuberculosis, rhinoscleroma (Klebsiella rhinoscleromatis), and necrotising invasive aspergillosis. On CT, the extent of irregular and circumferential tracheobronchial narrowing is clearly demonstrated, with an accompanying mediastinitis (opacification of the mediastinal fat) evident in some patients. In active disease, the narrowed trachea (and frequently a main bronchus) has an irregularly thickened wall. In the fibrotic or healed phase, the trachea is narrowed but has a smooth and normal thickness wall. They are uncommon, accounting for less than 1% of all thoracic malignancies. Most are squamous cell carcinomas and adenoid cystic carcinomas (Fig. 13-3). Other neoplasms, such as mucoepidermoid carcinoma, carcinoid tumour (Fig. 13-4), lymphoma, plasmocytoma and adenocarcinoma are rare. On CT, they appear as a soft-tissue mass, usually affecting the posterior and lateral wall (Fig. 13-3A). Often sessile and eccentric, resulting in asymmetric luminal narrowing, they are occasionally circumferential. They can be polypoid and mostly intraluminal, with mediastinal extension in 30–40%. The surface of tumour is often irregular in squamous cell carcinoma, whereas it is smooth in adenoid cystic carcinoma. Multiplanar reformation and volumetric rendering images are recommended for a precise pre-therapeutic assessment of extent (Figs. 13-3B, 13-4B and 13-4D). These tumours are best treated surgically (primary resection and re-anastomosis), followed by radiation. The large airways may be involved secondarily by malignant neoplasms as a result of either haematogenous metastasis or direct invasion from the oesophagus, thyroid, mediastinum or lung. Neoplasms that have a propensity to metastasise to the trachea and major bronchi include renal cell carcinoma and melanoma. On CT the abnormalities are usually focal and include intraluminal soft-tissue nodules and wall thickening (Fig. 13-5). The commonest benign neoplasms are hamartoma, leiomyoma, neurogenic tumour and lipoma. They are usually well demarcated, round and less than 2 cm in diameter, with typical radiological appearances of a smoothly marginated intraluminal polyp. Hamartomas and lipomas may demonstrate fat attenuation on CT. Tracheobronchial papillomatosis is a particular entity caused by human papillomavirus infection usually acquired at birth from an infected mother. The larynx is affected most commonly; extension into the trachea and proximal bronchi occurs occasionally. Exceptionally, the infection spreads into the lung parenchyma. The typical radiological findings consist of multiple small nodules projecting into the airway lumen or diffuse nodular thickening of the airway wall. Although benign, papilloma may undergo transformation to squamous cell carcinoma. Involvement of the large airways is a common manifestation of ANCA-associated granulomatous vasculitis (previously named Wegener granulomatosis). Inflammatory lesions may be present with or without subglottic or bronchial stenosis, ulcerations and pseudotumours. Radiological manifestations include thickening of the subglottic region and proximal trachea with a smooth symmetric or asymmetric narrowing over variable length. Stenosis may also be seen on any main, lobar or segmental bronchus, sometimes causing lobar or sublobar atelectasis. Nodular or polypoid lesions may also be seen on the inner contour of the airway lumen. Relapsing polychondritis is a rare systemic disease of autoimmune pathogenesis that affects cartilage at various sites, including the ears, nose, joints, and tracheobronchial tree. Histologically, the acute inflammatory infiltrate present in the cartilages and perichondrial tissue induces progressive dissolution and fragmentation of the cartilage followed by fibrosis. Symmetric subglottic stenosis is the most frequent manifestation in the chest. As the disease progresses, the distal trachea and bronchi may be involved. CT shows smooth thickening of the airway wall associated with more or less diffuse narrowing (Fig. 13-6). In the early stage, the posterior wall of the trachea is spared but in advanced disease circumferential wall thickening occurs (Fig. 13-7). The trachea may become flaccid with considerable collapse at expiration. Gross destruction of the cartilaginous rings with fibrosis may cause stenosis. Deposition of amyloid in the trachea and bronchi may be seen in association with systemic amyloidosis or as an isolated manifestation. As a result, the amyloid forms either multifocal or diffuse submucosal plaques or masses. The overlying mucosa is usually intact. Dystrophic calcification or ossification is frequently present. CT shows focal or, more commonly, diffuse thickening of the airway wall and narrowing of the lumen. Calcification may be seen. Narrowing of the proximal bronchi can lead to distal atelectasis, bronchiectasis, or both, obstructive pneumonia. Involvement of the trachea is rare, and when it occurs, it is associated with laryngeal involvement. The proximal and distal parts of the trachea may be affected and the appearance of the stenosis may be smooth, irregular and nodular, or even mass-like. Bronchial involvement is much more common as a manifestation of sarcoidosis. The commonest signs at CT are regular or nodular bronchial wall thickening reflecting the presence of granulomas and fibrous tissue in the peribronchial interstitium. This bronchial wall thickening may result in smooth or irregular bronchial narrowing, which correlates with the presence of mucosal thickening at bronchoscopy and presumably reflects prominent inflammation in this location. Obstruction of lobar or segmental bronchi may occur as a result of airway wall fibrosis, compression by granuloma or peribronchial lymph nodes, of conglomerate fibrosis or some combination of these phenomena. Bronchial stenosis may clear spontaneously or with steroid treatment. Ulcerative tracheitis and tracheobronchitis are rare complications and occur more often in association with ulcerative colitis than Crohn’s disease. In most but not all cases, the diagnosis of inflammatory bowel disease precedes the presence of airway disease. Histologically, tracheobronchitis is characterised by more or less concentric mucosal and submucosal fibrosis and chronic inflammation. Ulceration and luminal narrowing may be evident. Cartilaginous plates are not destroyed. On CT, the tracheobronchial walls are thickened and produce irregular luminal narrowing (Fig. 13-8). Bronchial wall thickening and bronchiectasis also may be present with or without mucoïd impaction. This rare disorder is characterised by the presence of multiple cartilaginous nodules and bony submucosal nodules on the inner surface of the trachea and proximal airways. Men are more frequently involved than women and most patients are more than 50 years on age. Histologically, the nodules contain heterotopic bone, cartilage and calcified acellular protein matrix. The overlying bronchial mucosa is normal and because it contains no cartilage, the posterior wall of the trachea is spared. The chest radiograph may be normal or may demonstrate lobar collapse or infective consolidation. If the tracheal air column is clearly seen, multiple sessile nodules that project into the tracheal lumen extending over a long segment of the trachea can be appreciated. CT shows thickened tracheal cartilage with irregular calcifications. The nodules may protrude from the anterior and lateral walls into the lumen; they usually show foci of calcification (Fig. 13-9). Characterised by a diffuse narrowing involving the intrathoracic trachea, this entity is almost always associated with COPD (Fig. 13-10). The pathogenesis of the lesion is obscure, but probably it is an acquired deformity related to the abnormal pattern and magnitude of intrathoracic pressure changes in COPD. On radiographs and CT, the condition is easily recognised by noting that the internal side-to-side diameter of the trachea is decreased to half or less than the corresponding sagittal diameter. On the postero-anterior radiograph and CT multiplanar reformations, the narrowing usually affects the whole intrathoracic trachea, with an abrupt return to normal calibre at the thoracic inlet (Fig. 13-10). The trachea usually shows a smooth inner margin but occasionally has a nodular contour. Tracheal cartilage calcification is frequently evident. It refers to patients who have marked dilatation of the trachea and mainstem bronchi. It is often associated with tracheal diverticulosis, recurrent lower respiratory tract infection and bronchiectasis. Atrophy affects the elastic and muscular elements of both the cartilaginous and membranous parts of the trachea. The diagnosis is based on radiological findings. The immediately subglottic trachea has a normal diameter, but it expands as it passes to the carina and this dilatation often continues into the major bronchi. Atrophic mucosa prolapses between cartilage rings and gives the trachea a characteristically corrugated outline on a plain radiograph. Corrugations may become exaggerated to form sacculations or diverticula. On CT a tracheal diameter of greater than 3 cm (measured 2 cm above the aortic arch) and diameter of 2.4 and 2.3 cm for the right and left bronchi, respectively, determine the diagnosis (Fig. 13-11). Additional findings include tracheal scalloping or diverticula (especially along the posterior membranous tracheal wall). Resulting from weakened tracheal cartilages, this abnormality may be seen in association with a number of disorders including tracheobronchomegaly, COPD, diffuse tracheal inflammation such as relapsing polychondritis, as well as following trauma. On the radiographs, a reduction by almost 75% of the sagittal diameter at expiration is an excellent indicator of the diagnosis. The increase in compliance is due to the loss of integrity of the wall’s structural components and is particularly associated with damaged or destroyed cartilages. The coronal diameter of the trachea becomes significantly larger than the sagittal one, producing a lunate configuration to the trachea.9 The flaccidity of the trachea or bronchi is usually most apparent during coughing or forced expiration. In patients with COPD with high downstream resistance particularly high dynamic pressure gradients can be generated across the tracheal wall and it is likely that calibre changes of more than 50% can occur at expiration with normal tracheal compliance. As a result only a decrease in cross-section area of the tracheal lumen greater than 70% at expiration indicates tracheomalacia. Dynamic expiratory multislice CT may offer a meaningful alternative to bronchoscopy in patients with suspected tracheobronchomalacia10,11 because of its possibilities to provide morphological and functional information simultaneously. Dynamic expiratory CT may show complete collapse or collapse of greater than 75% of luminal cross-section (Fig. 13-12). Involvement of the central tracheobronchial tree may be diffuse or focal. The reduction of airway may result in an oval or crescent shape. The crescent shape is due to the bowing of posterior membranous trachea. Multidetector CT (MDCT) with thin collimation is the most accurate technique for identifying peripheral bronchopleural fistula most commonly caused by necrotising pneumonia or secondary to traumatic lesions. Nodobronchial and nodobroncho-oesophageal fistulas most commonly caused by Mycobacterium tuberculosis infection are depicted by the presence of gas in cavitated hilar or mediastinal lymph adenopathy adjacent to the airways. Tracheal diverticula and tracheobroncho-oesophageal fistula may also be diagnosed even in adults. Malignant neoplasia, particularly oesophageal, is the most common cause of tracheo-oesophageal fistulas in adults. Occasionally congenital fistulas are first manifested in adults. Infection and trauma are the most frequent non-malignant causes. MDCT has a high degree of sensitivity and specificity for depicting bronchial anastomotic dehiscence occurring after lung transplantation. Bronchial dehiscence is seen as bronchial wall defect associated with extraluminal air collections. Bronchiectasis is a chronic condition characterised by local, irreversible dilatation of bronchi, usually associated with inflammation. Despite its decreased prevalence in developed countries, bronchiectasis remains an important cause of haemoptysis and chronic sputum production. Although the causes of bronchiectasis are numerous, there are three mechanisms by which the dilatation can develop: bronchial obstruction, bronchial wall damage and parenchymal fibrosis (Table 13-1). In the first two mechanisms, the common factor is the combination of mucus plugging and bacterial colonisation. Cytokines and enzymes released by inflammatory cells plus toxins from the bacteria result in a vicious cycle of increasing airway wall damage, mucus retention and bacterial proliferation. In the case of parenchymal fibrosis, the dilatation of bronchi is caused by maturation and retraction of fibrous tissue located in the parenchyma adjacent to an airway (traction bronchiectasis). TABLE 13-1 Mechanisms and Causes of Bronchiectasis Pathologically, bronchiectasis has been classified into three subtypes, reflecting increasing severity of disease: cylindrical, characterised by relatively uniform airway dilatation; varicose, characterised by non-uniform and somewhat serpiginous dilatation; and cystic. As the extent and degree of airway dilatation increase, the lung parenchyma distal to the affected airway shows increasing collapse of fibrosis. CXR reveals abnormalities in the majority of cases. Thickened bronchial walls are visible either as single thin lines or as parallel line opacities (tramline) (Fig. 13-13). When seen end-on, bronchiectatic airway appears as poorly defined ring or curvilinear opacities. Dilated bronchi filled with mucus or pus result in tubular or ovoid opacities of variable size. Cystic bronchiectasis manifests as multiple thin-walled ring shadows often containing air–fluid levels (Fig. 13-14). Pulmonary vessels may appear increased in size and may be indistinct because of adjacent peribronchial inflammation and fibrosis. In generalised bronchiectasis, such as that associated with cystic fibrosis, overinflation is often present. Localised forms are frequently accompanied by atelectasis which may be mild and detected only because of vascular crowding, fissure displacement or obscuration of part of the diaphragm. The major sign of bronchiectasis on thin collimation CT (high-resolution CT, HRCT) is dilatation of the bronchi, either with or without bronchial wall thickening (Figs. 13.15–13.19). CT shows bronchial dilatation including lack of tapering of bronchial lumina (the cardinal sign of bronchiectasis), internal diameter bronchi greater than that of the adjacent pulmonary artery (signet ring sign), visualisation of bronchi within 1 cm of the costal pleura or abutting the mediastinal pleura, and mucus-filled dilated bronchi. In varicose bronchiectasis, the bronchial lumen assumes a beaded configuration. Cystic bronchiectasis is seen as a string of cysts caused by sectioning irregular dilated bronchi along their lengths, or a cluster of cysts, caused by multiple dilated bronchi lying adjacent to each other. Cluster of cysts are most frequently seen in atelectatic lobes. Air–fluid levels, caused by retained secretion may be present in the dependent portion of the dilated bronchi. Secretion accumulation within bronchiectatic airways is generally easily recognisable as lobulated glove-finger, V- or Y-shaped densities (Fig. 13-16). When oriented perpendicular to the CT slice, filled dilated bronchi are visualised as nodular opacities running alongside adjacent pulmonary arteries (whose diameters are smaller than those of the dilated bronchi). CT may show complete collapse of the lobe containing bronchiectatic airways. Subtle degrees of volume loss may be seen in lobes in early disease. This is most evident in the lower lobes on the basis of crowding of the mildly dilated bronchi and posterior displacement of the oblique fissure (Fig. 13-15).
Airway Disease and Chronic Airway Obstruction
Introduction
Tracheal Disorders1–8
Post-Traumatic Strictures7
Infectious Tracheobronchitis3–7
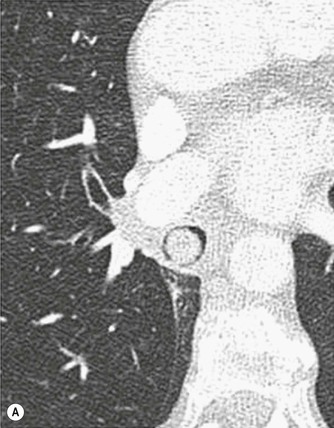
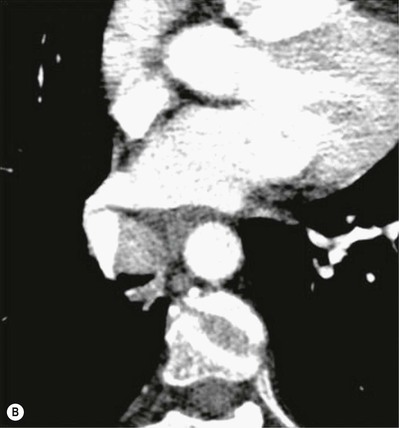
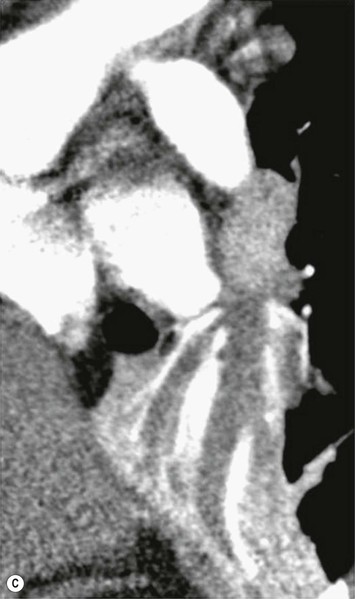
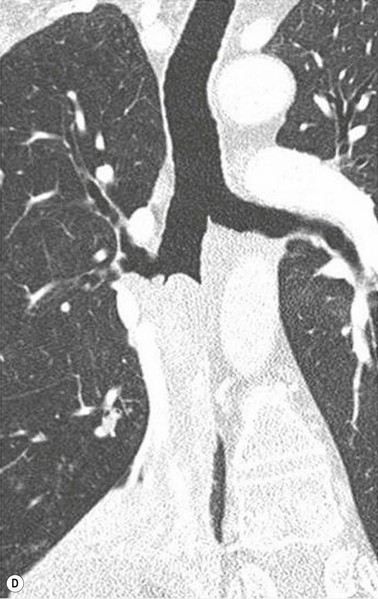
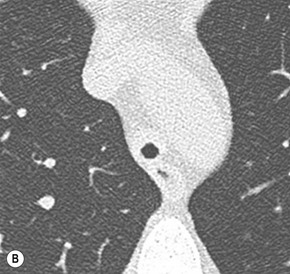
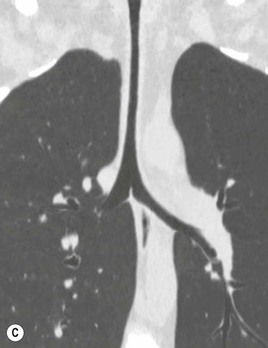
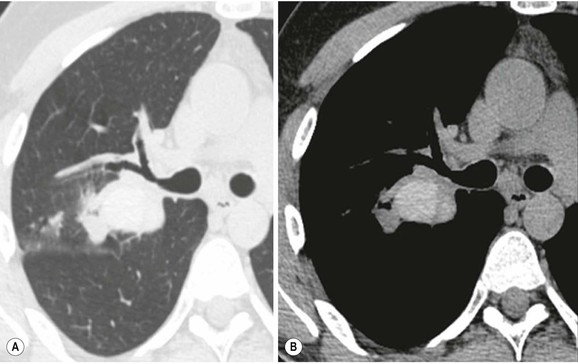
Primary Malignant Neoplasms2–4,6,8
Secondary Malignant Neoplasms2–4,6,8
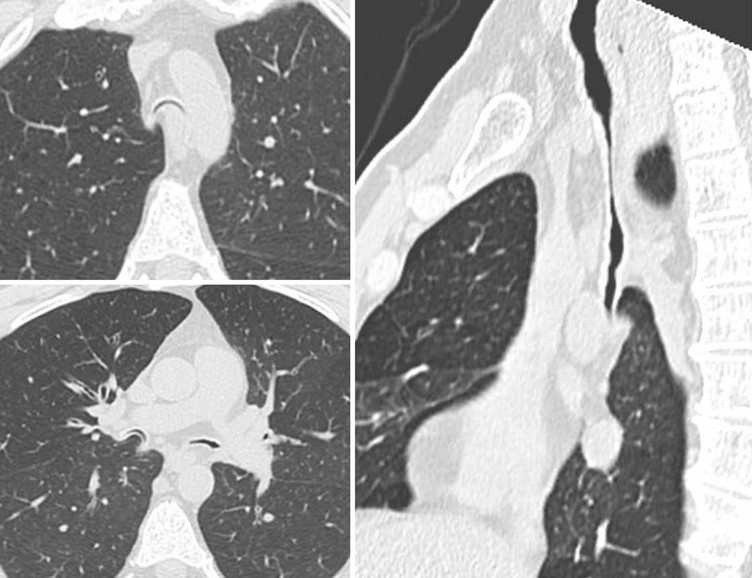
Benign Neoplasms2–4,6,8
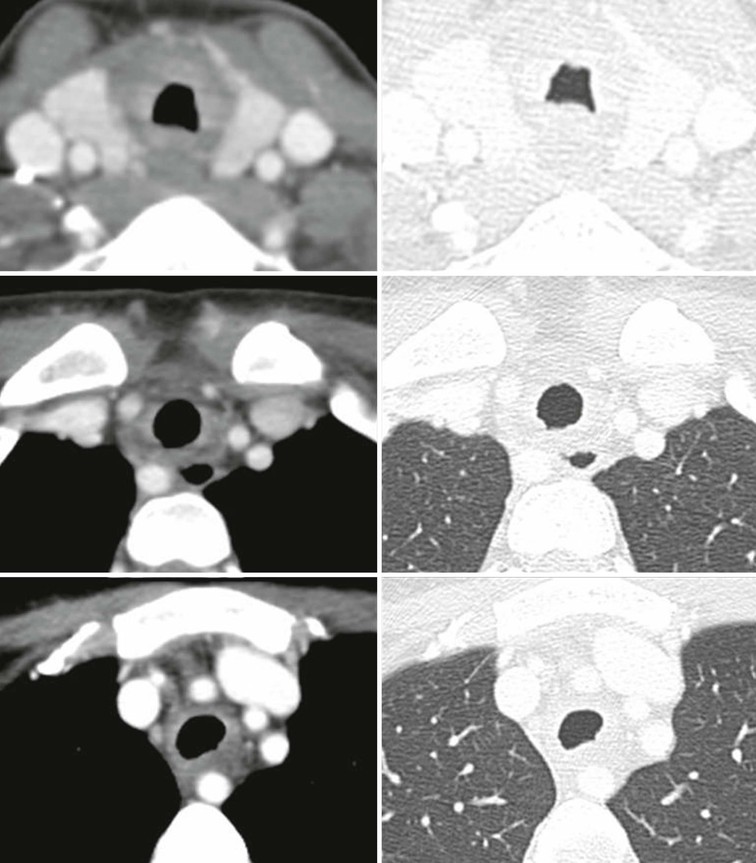
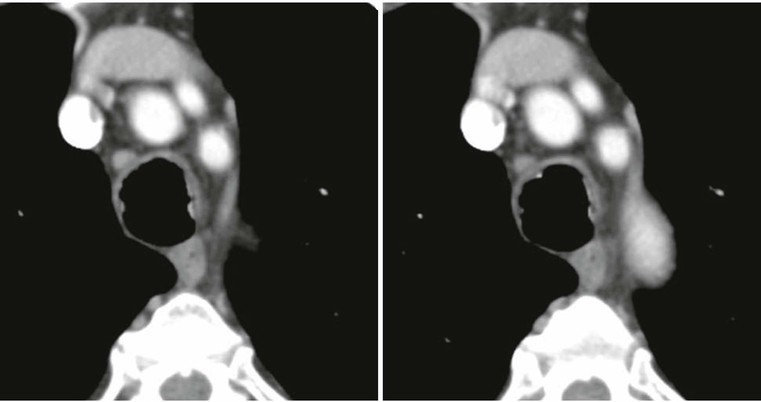
ANCA-Associated Granulomatous Vasculitis2,3,7
Relapsing Polychondritis2,3,7
Tracheobronchial Amyloidosis2,3,7
Sarcoidosis2,3,6,7
Inflammatory Bowel Disease2,3,7
Tracheobronchopathia Osteochondroplastica2,3
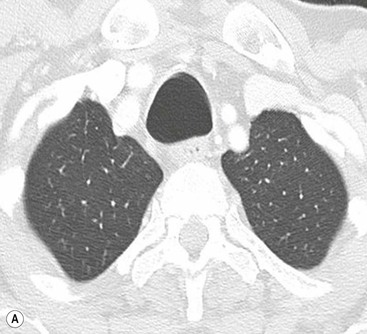
Sabre-Sheath Trachea2,3,6,7
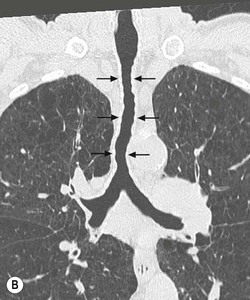
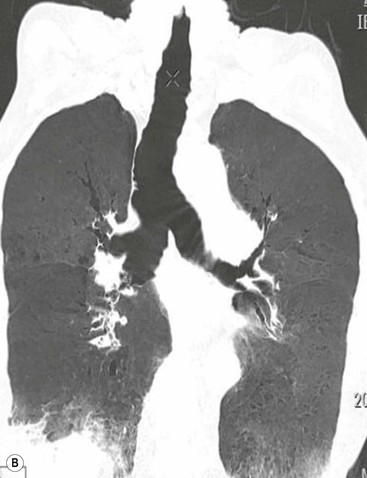
Tracheobronchomegaly (Mounier–Kuhn Syndrome)2,3,6,7
Tracheobronchomalacia3,7,9
Tracheobronchial Fistula and Dehiscence2,3,5
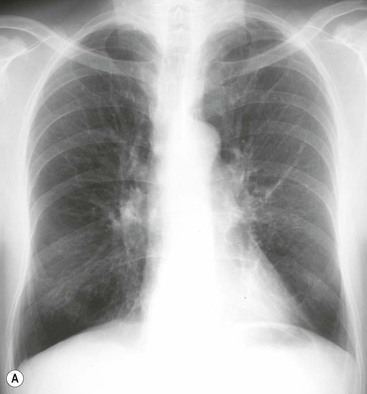
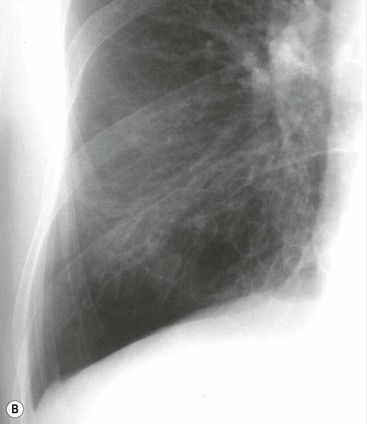
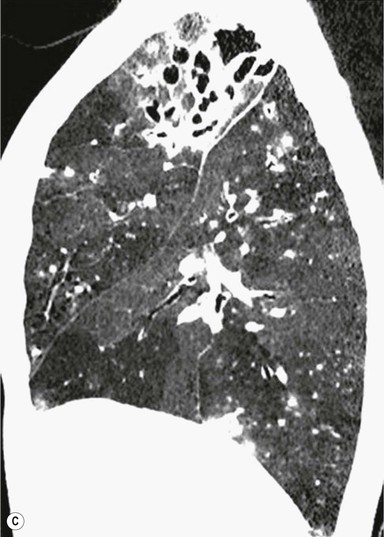
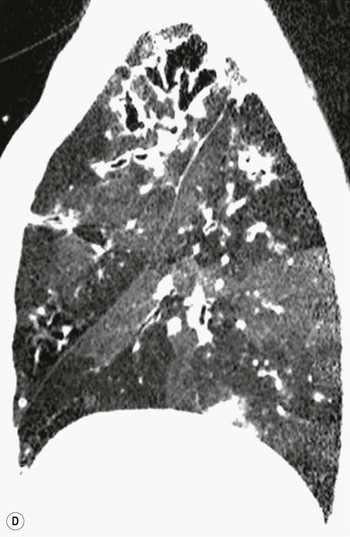
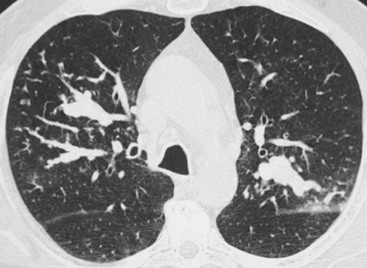
Bronchiectasis2–6,12–14

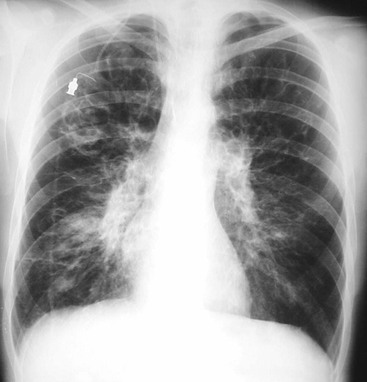
Radiographic Findings
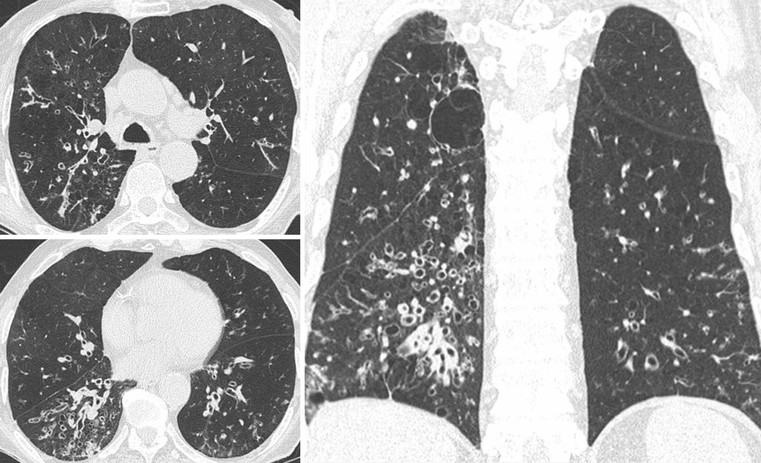
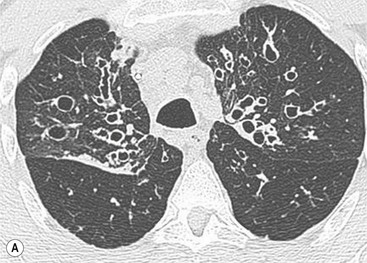
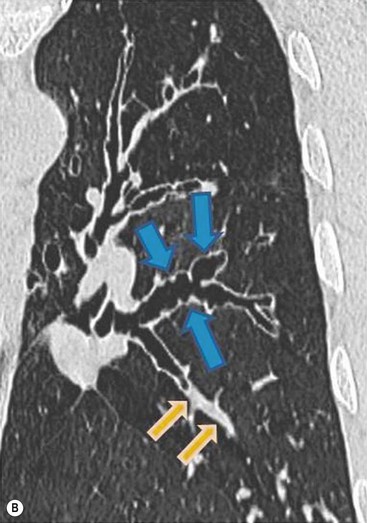
CT Findings