11 Airways, Lungs, Pleurae, Mediastinum, Diaphragm, and Chest Wall The final step of the chest radiographic interpretation is to assess the abnormalities in the airways, lung parenchyma, pleurae, mediastinum, diaphragm, and bones and soft tissue of the chest wall. For example, this part of the report may read: “The left lower lobe is collapsed, which may result from compression of the left lower lobar bronchus by an enlarged left atrium or direct compression of this part of the lung by the enlarged and displaced left ventricle. The collapsed left lower lobe obliterates the left diaphragmatic contour. There are small amounts of pleural effusion in both thoraces. The sternum is mildly bowed forward by the enlarged heart.” Abnormalities of the airways and lungs are common in patients with cardiovascular disease (Table 11.1). These abnormalities may be due to a mechanical effect by enlarged or abnormally positioned cardiovascular structures on adjacent airways or lung parenchyma. Less commonly, the abnormalities of the airway or lungs may be associated with congenital heart disease without any obvious causal relationship.
 Airways and Lungs
Airways and Lungs
| Airway compression | Aorta or its branches |
| – Dilated aortic arch | |
| – Posterior displacement of the ascending aorta and aortic arch | |
| – Long transverse aortic arch | |
| – Low lying aortic arch | |
| – Innominate artery compression syndrome | |
| – Vascular ring or sling | |
| – Aortic diverticulum of Kommerell | |
| Pulmonary arteries | |
| – Dilated pulmonary arteries with left-to-right shunt | |
| – Dilated branch pulmonary arteries in pulmonary hypertension | |
| – Absent pulmonary valve syndrome | |
| – Displacement of a pulmonary artery | |
| Cardiac chambers | |
| – Dilated left atrium | |
| Secondary to small thoracic cage | |
| Intrinsic airway abnormality | Abnormal branching pattern |
| – Tracheal bronchus | |
| – So-called bridging bronchus | |
| Intrinsic stenosis of the trachea or a bronchus | |
| Tracheomalacia and bronchomalacia | |
| Tracheal and bronchial calcifications | Idiopathic calcification in children |
| Warfarin sodium administration | |
| Adrenogenital syndrome | |
| Skeletal dysplasias | |
| – Chondrodysplasia punctata | |
| – Diastrophic dysplasia | |
| – Hydrops ectopic calcification, moth-eaten skeletal dysplasia | |
| Lung collapse | Extrinsic airway compression |
| Endobronchial mucous plug or retention of secretions | |
| Direct compression of the lung | |
| Emphysema | Extrinsic airway compression |
| Endobronchial mucous plug or retention of secretions | |
| Diffuse interstitial edema | |
| Compensatory emphysema | |
| Small airway disease | |
| Consolidation | Pneumonia complicating already compromised ventilation |
| Pneumonia secondary to frequent aspiration | |
| Pulmonary hemorrhage | |
| Pulmonary infarction | |
| Lung hypoplasia | Scimitar syndrome |
| Unilateral absence of a pulmonary artery | |
| Unilateral stenosis of a pulmonary artery | |
| Unilateral pulmonary vein atresia/stenosis | |
| Isolated | |
| Horseshoe lung | Associated with scimitar syndrome |
| Isolated |
Airway Compression
The major airways are neighbored by the aortic arch, branch pulmonary arteries, and left atrium. Abnormal positions and dilatation of any of these structures may compress the trachea or main bronchi. A classic airway compression is due to vascular rings and slings.1 Infrequently, the airway may be compressed by a normally formed but abnormally positioned vascular structure.2,3 The severity of airway compression by cardiovascular structures depends not only on the vascular anatomy but also on the size and shape of the thoracic cage.
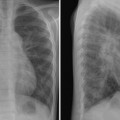
Tracheal compression is typically seen in aortic arch anomalies causing a vascular ring.1,4 The tracheal and esophageal compression is most obvious and consistent when there is a double aortic arch (Fig. 11.1). A right or left aortic arch with an aberrant subclavian or innominate artery characteristically causes compression of the esophagus due to its retroesophageal course in front of the spine. A posterior indentation of the trachea can be obvious when the aberrant artery arises from the descending aorta through a large diverticulum of Kommerell (Fig. 11.2). A similar but more severe indentation can be seen with so-called circumflex retroesophageal aortic arch (Fig. 11.3).5 Tracheal narrowing can be minimal or absent when the diverticulum is small or absent. The trachea may be compressed by the ascending aorta and aortic arch that have a more posterior position or by the aortic arch that has a long transverse course in front of the trachea (Fig. 11.4).2,3 The innominate artery as well may compress the distal trachea when it arises more distally from the aortic arch on the left side and crosses the midline in front of the trachea.6 This condition has been described as innominate artery compression syndrome. Nonetheless, the pretracheal location of the innominate artery is often seen but rarely symptomatic.7
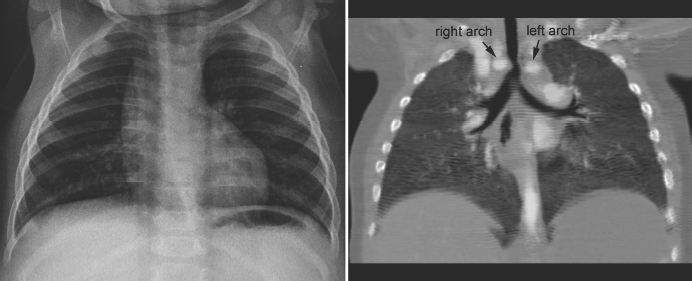
Fig. 11.1 Double aortic arch causing narrowing of the distal trachea. The right aortic arch is larger than the left arch and makes a sharp indentation of the trachea on the right side. Airway narrowing is obvious on the frontal chest radiograph but this finding is not specific for double aortic arch.
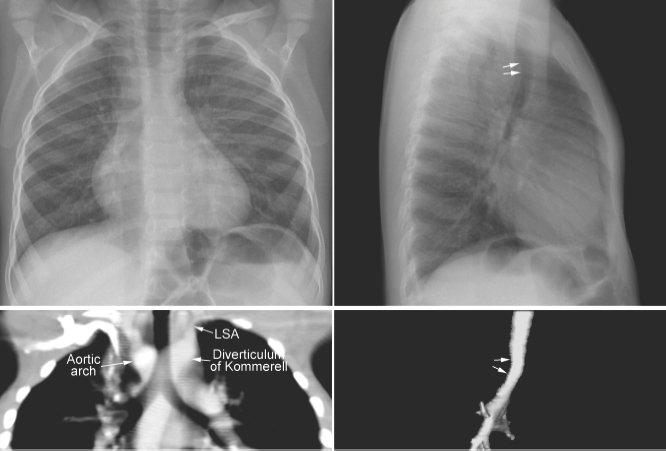
Fig. 11.2 Right aortic arch with aberrant origin of the left subclavian artery (LSA) from the descending aorta through the diverticulum of Kommerell. The distal trachea shows mild narrowing. The trachea is not significantly deviated to the left but it is bent forward by the distal aortic arch and diverticulum (arrows on the lateral views).
Tracheal compression and narrowing may be identified on the frontal and lateral chest radiographs. Severe tracheal narrowing may be associated with emphysema of both lungs, especially with tracheomalacia. As air trapping develops, the hyperinflated lungs displace the ascending aorta and aortic arch further backward, causing a vicious cycle of airway obstruction and emphysema.
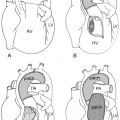
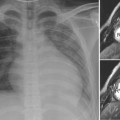
As the left main bronchus is normally crossed over by the aortic arch and left pulmonary artery, dilatation of those vessels may cause left bronchial compression from above (Figs. 11.5, 11.6). The left main bronchus is more vulnerable to compression when the aortic arch has an unusually low position. The most proximal part of the left main bronchus is also close to the proximal right pulmonary artery. Therefore, the left main bronchus may be compressed by the dilated right pulmonary artery. The right main bronchus is transversely positioned in the mediastinum behind the right pulmonary artery and in front of the spine. A dilated right pulmonary artery may therefore compress both main-stem bronchi (Fig. 11.6). As the ascending aorta is immediately in front of the right pulmonary artery, dilatation or an abnormal posterior position of the ascending aorta may cause or contribute to right main bronchial compression. A proximal descending aorta on the right side seen with a right or double aortic arch may also compress the right main bronchus. Significant compression of the main-stem bronchi is almost always seen when there is severe dilatation of the branch pulmonary arteries with absent pulmonary valve syndrome (Fig. 11.6) and less commonly with more modest dilatation in pulmonary arterial hypertension.
The left main bronchus or left lower lobar bronchus may also be obstructed by a markedly dilated left atrium (Figs. 11.7, 11.8). A dilated left atrium typically displaces and compresses the left main bronchus and its lower lobar bronchus backward and upward, causing left lung or left lower lobe collapse (Fig. 11.7).
Patients with congenital heart disease with large left-to-right shunts often develop wheezing with obstructive emphysema or atelectasis (Figs. 11.9, 11.10). As the bronchi and pulmonary arteries course together in the lungs, dilatation of the pulmonary arteries causes some degree of bronchial compression. This may be further complicated by any insult to the respiratory system, causing significant symptoms and signs of airway obstruction.
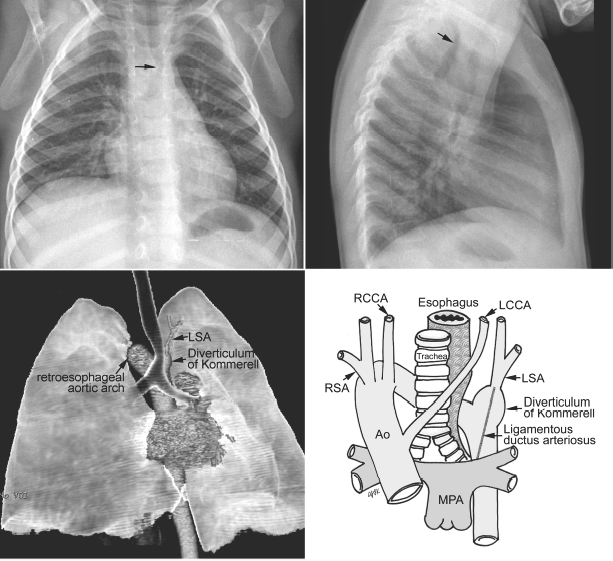
Fig. 11.3 Circumflex retroesophageal right aortic arch. The proximal part of the aortic arch is on the right side, indenting the right tracheal wall (arrow on the frontal chest radiograph). The aortic arch then takes a long oblique downward course behind the trachea and esophagus to connect to the descending aorta on the left. The trachea shows forward bending by the retroesophageal part of the aortic arch (arrow on the lateral chest radiograph). In the majority of cases, the left subclavian artery (LSA) arises from the top of the descending aorta through the diverticulum of Kommerell. Ao, ascending aorta; LCCA, left common carotid artery; MPA, main pulmonary artery; RCCA, right common carotid artery; RSA, right subclavian artery.
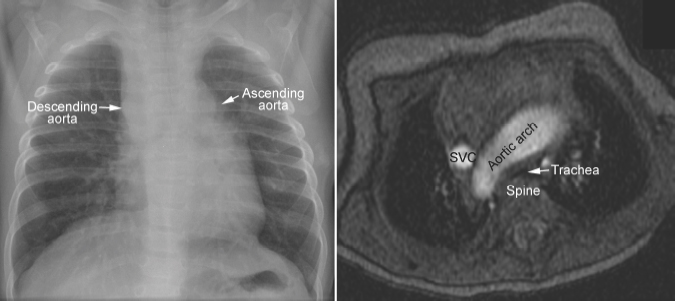
Fig. 11.4 Right aortic arch in corrected transposition of the great arteries. The aorta ascends on the left side, forming a convex bulge of the left upper mediastinal border. As the ascending aorta and descending aorta are on the opposite sides of the mediastinum, the aortic arch has a long transverse course in front of the trachea, causing compression of the trachea against the spine. SVC, superior vena cava.
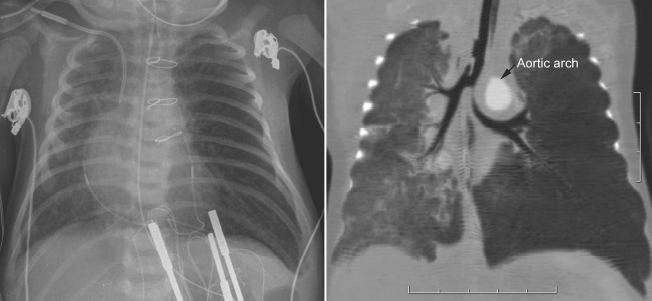
Fig. 11.5 Left main bronchial compression by an augmented aortic arch and surrounding edema in a newborn with complete transposition of the aorta and aortic arch narrowing. The left lung is hyperinflated.
Patients with frequent episodes of aspiration may show patchy lung parenchymal consolidation or atelectasis in the lower lobes. Patients who aspirate in the supine position may show lung opacities in the upper lobes and superior segments of the lower lobes.
Intrinsic Abnormalities of the Airway
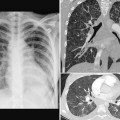
Airway narrowing can also be due to inadequate rigidity of the tracheal or bronchial wall causing tracheomalacia or bronchomalacia, which can develop in utero or anytime after birth (Fig. 11.8).8 Tracheomalacia may be due to primary intrinsic weakness of the tracheal cartilage but more commonly is a result of chronic extrinsic compression by an abnormal vessel such as a vascular rings or pulmonary artery sling. In addition, narrowing of the airway may be due to an intrinsic stenosis from complete cartilaginous rings, typically seen with pulmonary artery sling (Fig. 11.11).9,10
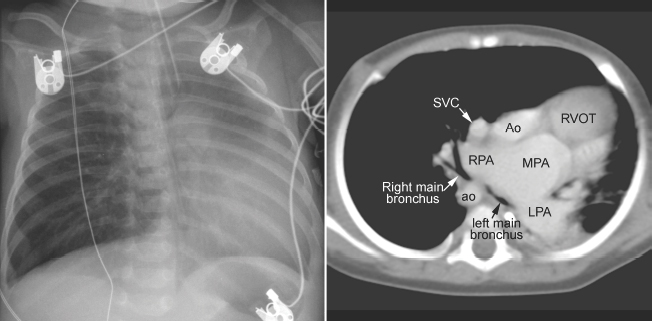
Fig. 11.6 Severe hyperinflation of the right lung with compression of the proximal right main bronchus between the dilated right pulmonary artery (RPA) and right-sided descending aorta (ao) in a patient with tetralogy of Fallot, absent pulmonary valve syndrome, and right aortic arch. The hyperinflated right lung herniates into the left thorax and displaces the heart and mediastinal structures leftward and backward. Most of the left lung is collapsed. Airway compression and hyperinflation can result in a vicious cycle and rapid deterioration. As hyperinflation secondary to airway compression pushes the heart and mediastinal structures backward, the main bronchi are further compressed against the spine and descending aorta. Ao, ascending aorta; LPA, left pulmonary artery; MPA, main pulmonary artery; RVOT, right ventricular outflow tract; SVC, superior vena cava.
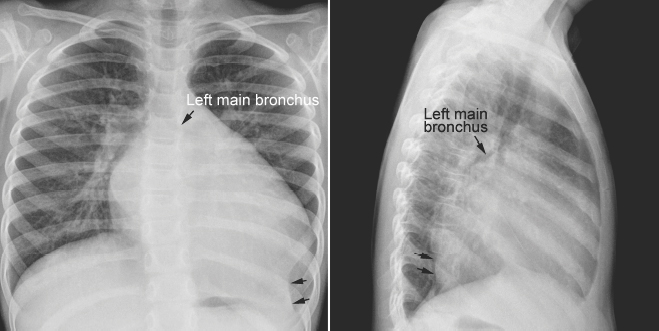
Fig. 11.7 Left lower lobe collapse in a 3-year-old child with dilated cardiomyopathy. The heart is markedly enlarged with a dilated left atrium and left ventricle. The left main bronchus is displaced upward and backward. The left lower lobe is collapsed (arrows). The left diaphragmatic silhouette is obliterated. There is compensatory hyperinflation of the left upper lobe.
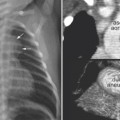
An abnormal branching pattern of the airway can be recognized on well-taken chest radiographs. Abnormal bronchial branching patterns in situs inversus and visceral heterotaxy with right or left thoracic and atrial isomerism are discussed in Chapter 7. A tracheal bronchus is the most common form of abnormal branching of the airway in which an abnormal bronchus arises from the lateral wall of the trachea and supplies the entire or a part of the upper lobe, more commonly on the right than the left.11 Bilateral tracheal bronchi have rarely been described. Tracheal bronchus is usually asymptomatic and found as an incidental finding on an imaging study or bronchoscopy but can be associated with chronic atelectasis, recurrent infection, or bronchiectasis. The main clinical implication of tracheal bronchus is during endotracheal intubation (Fig. 11.12).12,13 The tracheal bronchus can be occluded by an endotracheal or tracheostomy tube, resulting in persistent atelectasis of the affected upper lobe. Inadvertent intubation of the tracheal bronchus results in regional variation in ventilation and may be complicated by pneumothorax with mechanical ventilation. There is an apparent increased incidence of tracheal bronchus in Down syndrome. An abnormal branching pattern of the tracheobronchial tree is seen in 40 to 80% of cases with pulmonary artery sling (Fig. 11.11).9,10 Typically the trachea bifurcates into the main-stem bronchi at a lower level than normal, with a wide angle between the right and left bronchi, producing an inverted-T appearance. In approximately one third of the cases with a low inverted-T bifurcation of the airway, the right upper lobar bronchus arises from the trachea slightly above the level of normal tracheal bifurcation. The left pulmonary artery forms a sling on the right side of the airway immediately above the lower bifurcation. The low inverted-T pattern of airway bifurcation has previously been described as a bridging bronchus.9 Almost all cases with a low inverted-T bifurcation of the airway, with or without a separate origin of the right upper lobe bronchus, are associated with long segment narrowing of the lower airway above the bifurcation. The narrowing is due to complete cartilaginous rings with absence of the membranous part of the trachea posteriorly.
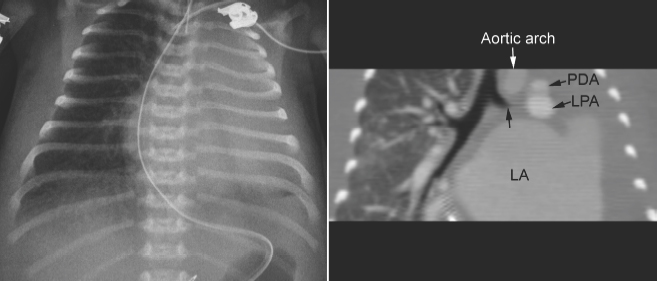
Fig. 11.8 Left lung collapse due to complete obstruction of the left main bronchus in a 6-day-old patient with critical aortic stenosis and severe mitral regurgitation. Computed tomography (CT) angiogram shows complete obstruction of the left main bronchus (arrow). Although the left atrium is hugely dilated and certainly compresses the left main bronchus, the level of proximal obstruction is at some distance from the site of left atrial compression. The patient was considered to have bronchomalacia. LA, left atrium; LPA, left pulmonary artery; PDA, patent ductus arteriosus.
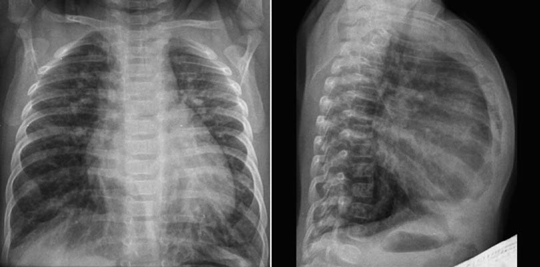
Fig. 11.9 Hyperinflation of both lungs in an infant with ventricular septal defect. The heart is moderately enlarged and the pulmonary vascularity is markedly increased. The diaphragm does not form a clear contour on the frontal view because it is flattened by the hyperinflated lungs. The sternum is bowed forward due to both cardiomegaly and hyperinflation.
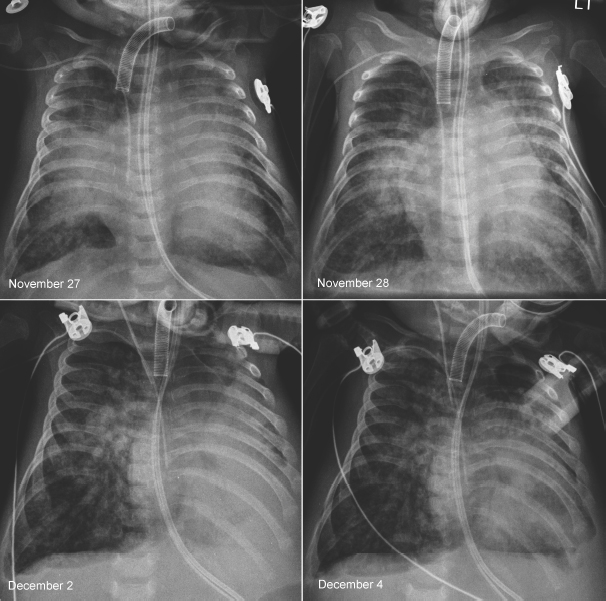
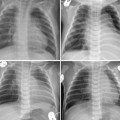
Fig. 11.10 Rapidly changing lung ventilation in a 3-month-old neonate with atrioventricular septal defect. The heart is moderately enlarged and the pulmonary vascularity is markedly increased. On the first radiograph on November 27, the right middle lobe and the upper part of the left upper lobe were collapsed. On the following day, the right middle and left upper lobes showed better aeration. Five days later, the left lower lobe and the lingular segment of the left upper lobe are collapsed and the right lung is hyperinflated. On the final radiograph, the left lower lobe showed improved aeration, whereas the right lung showed persistent hyperaeration. There was poor correlation between the patient’s clinical findings and radiographic changes.
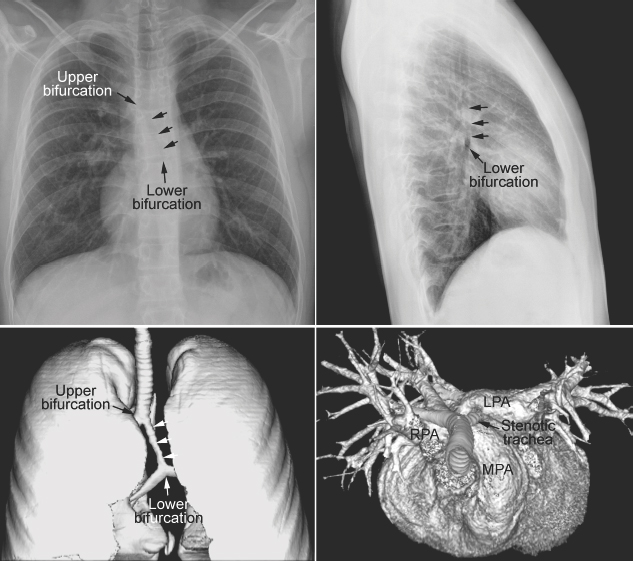
Fig. 11.11 Abnormal bronchial branching and congenital tracheal stenosis in pulmonary artery sling. There are two bifurcations of the airway in the mediastinum. The upper bifurcation gives rise to a bronchus to the right upper lobe. The lower bifurcation is into the left main bronchus and the bronchus to the right middle and lower lobes. The airway between the two bifurcations is diffusely narrowed due to complete cartilaginous rings (arrows). The three-dimensional CT angiogram as seen from above and front shows that the left pulmonary artery (LPA) arises far distally from the right pulmonary artery (RPA), encircling the right side of the stenotic trachea and then coursing leftward behind the trachea to reach the left hilum. MPA, main pulmonary artery.
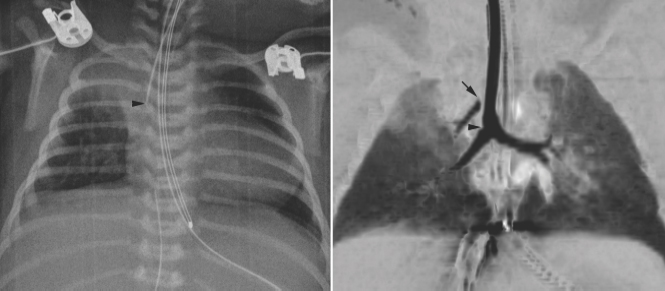
Fig. 11.12 Right upper lobe collapse in a newborn with truncus arteriosus. The endotracheal tube tip (arrowhead) is close to the carina. The right upper lobe is collapsed. Subsequent CT scan showed a tracheal bronchus supplying the collapsed right upper lobe. The orifice of the tracheal bronchus was occluded (arrow) by the endotracheal tube.
Warfarin Sodium-Induced Calcification of Tracheal and Bronchial Cartilaginous Rings
Progressive calcification of the cartilaginous rings of the trachea and bronchi has been observed in up to 50% of the patients undergoing prolonged anticoagulant therapy with warfarin sodium.14 The calcification appears linear or beaded along the wall of the trachea and major bronchi on chest radiographs (Fig. 11.13). Other causes of airway calcifications are listed in Table 11.1.
Collapse, Emphysema and Consolidation
Segmental or lobar collapse involving lower lobes is common when the heart is enlarged. Collapse can be due to mechanical compression of a main, lobar, or segmental bronchus by the enlarged heart or dilated vessel (Figs. 11.7, 11.8, 11.14). The left lower lobe is most commonly affected especially when the left atrium is dilated (Fig. 11.7). A completely collapsed lobe or segment is seen as a triangular hazy area without visible vascular or bronchial markings. The base of the triangular haziness is on the pleural surface, whereas its apex is directed toward the lung hilum. A poorly ventilated lung area is characterized by crowded vascular markings. Collapse can also be due to retention of secretions or a mucus plug in an already narrowed bronchus or bronchi. In addition the enlarged heart may directly compress the lung parenchyma. A poorly ventilated lung area due to direct compression may show air-bronchograms as well as crowded vascular markings. In contrast, a pneumonic consolidation typically does not show significant loss of lung volume.
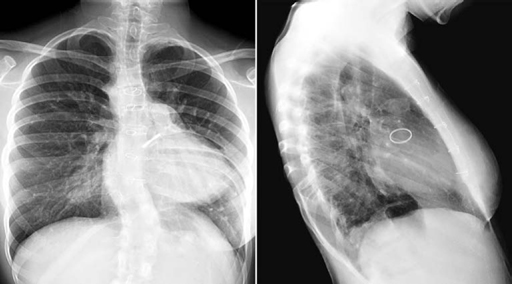
Fig. 11.13 Warfarin sodium-induced calcification of the trachea and bronchi in a 16-year-old girl. The patient underwent repair of truncus arteriosus followed by replacement of the regurgitant truncal valve in the aortic position with a mechanical valve. She was on prolonged administration of warfarin sodium for her mechanical aortic valve. The walls of the trachea and major bronchi are lined with beaded calcification.
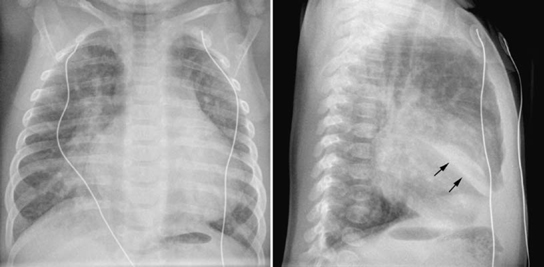
Fig. 11.14 Right middle lobe collapse in a 5-week-old infant with ventricular septal defect. The pulmonary vascularity is moderately increased. The medial segment of the right middle lobe is collapsed. On the frontal view, ill-defined haziness is seen in the medial aspect of the right lower lung partially obliterating the right lower cardiac border. On the lateral view, a fusiform dense haziness is seen in the lower aspect of the right middle lobe area. The inferior margin of the haziness is sharply demarcated by the major fissure (arrows). Loculated pleural effusion in the major fissure may show a similar finding.
The lungs are hyperinflated when the airways are partially obstructed by extrinsic vascular compression, as discussed earlier (Figs. 11.5, 11.6, 11.9, 11.10). Hyperinflation is common when heart disease is complicated by pulmonary edema. Hyperinflation is primarily due to increased resistance to gas flow through the edematous small airway, but reflex bronchoconstriction is also considered to play a role.15 The most typical combination of interstitial edema and hyperinflation is seen in newborns with an obstructive type of total anomalous pulmonary venous connection (Fig. 11.15). Hyperinflation can also be due to associated small airway disease such as bronchiolitis or asthma (Fig. 11.16). On a frontal chest radiograph, the lung volumes are increased with downward displacement of the diaphragms. When the displaced diaphragm is flat or convex downward, the diaphragmatic contour on the frontal view is blurred (Figs. 11.9, 11.14). In addition, the cardiac silhouette appears to be floating above the diaphragm, which is pushed downward by the hyperinflated lower lobes. The flattened or inverted diaphragm can also be readily appreciated on lateral radiographs. Severe hyperinflation of the lungs results in increased anteroposterior diameter of the thorax (Fig. 11.9). Hyperinflation of the lungs is often associated with scattered areas of subsegmental, segmental, or lobar atelectasis (Fig. 11.10). Not uncommonly, the collapsed lung areas become hyperinflated and the hyperinflated areas become collapsed in a short interval, and therefore the radiographs may appear quite different from day to day without any significant change in clinical findings. Finally, severely cyanotic patients can develop compensatory hyperinflation of the lungs due to reflex hyperventilation.
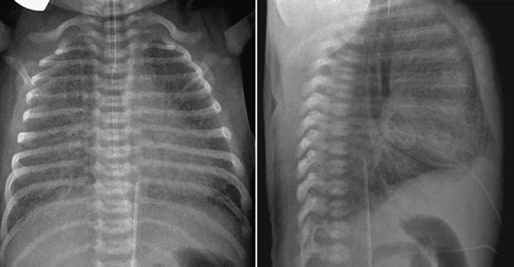
Fig. 11.15 Hyperinflation and interstitial edema of both lungs in a 2-day-old newborn with total anomalous pulmonary venous connection to the portal vein. Both lungs show generalized increase in interstitial markings. Both lung volumes are increased with flattening of the diaphragm.
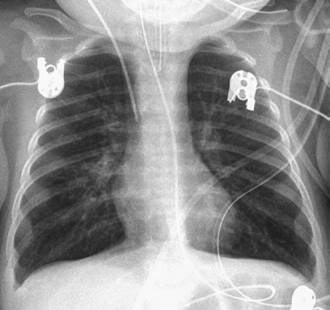
Fig. 11.16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree