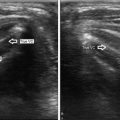
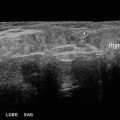
Fig. 35.1
US-guided ethanol ablation of a thyroid cyst. Basal US image of a left lobe thyroid cyst (B-mode, transverse view) (a). Needle insertion into the cyst using a needle track (b). Once the needle has been inserted, the needle track is removed from the screen image (c). Aspiration of fluid content of the cyst—the cyst shrinks—(d). Ethanol instillation—ethanol is visible as hyperechoic material—(e). The so called “mirror image” artifact of the needle can be observed. The thyroid cyst at the end of the procedure (f)
35.2.1.1 Needle Insertion
The needle may be either introduced through a guiding device connected to the US transducer (Fig. 35.1b), or alternatively, inserted free-hand and cautiously directed into the lesion with continuous US imaging and probe adjustment to maintain the needle in the field of view (Fig. 35.1c). Neither approach is superior to the other in terms of results: however, the free-hand procedure is more widespread and offers the convenience of repositioning the needle along a wider range of spatial axes throughout the maneuver, a valuable option when treating large and complex cysts.
35.2.1.2 Drainage of the Fluid Content
After extraction of the needle stylet, a 20 ml syringe is connected to the needle for fluid drainage and collection. The use of two devices may quite effectively facilitate this phase: a syringe holder (e.g., the Cameco pistol) and a catheter (20–25 cm length) connecting the needle and the syringe. Both devices contribute to facilitating drainage of the fluid content, as well as the subsequent alcohol instillation; at the same time, the connecting tube prevents the transmission of abrupt traction and strain movements to the needle (Fig. 35.2). This is particularly relevant if we consider that the change of shape and size of the cyst during fluid drainage may result in needle tip mispositioning that requires correction by the operator. The aspiration of the cystic fluid should be slow, smooth and constant in order to minimize the risk of ex-vacuum hemorrhaging. Obviously, large cysts may require the use of more than 1–2 syringes. All the materials likely to be needed for each procedure should be readily available.


Fig. 35.2
Equipment for the ethanol ablation of a thyroid cyst (from the left): a 20- or a 22-gauge spinal needle with stylet; 2 % lidocaine for local anesthesia (seldom necessary); 95 % sterile alcohol; saline solution for washing the needle at the end of the procedure; a 20 mL syringe for alcohol injection; a 5 mL syringe for the administration of lidocaine in case of local anesthesia; a flexible extension tube (bottom) for connecting the hub of the needle to the tip of syringe
35.2.1.3 Ethanol Instillation
The 95 % sterile ethanol, usually contained in 10 ml syringes, is cautiously injected into the cyst. Other sclerosing agents that have been proposed for thyroid cysts are polydecanol [16], arginine hydrochloride [17], and sodium tetradecyl sulfate [18]; however none of these substances has proved to be superior to alcohol. During instillation, ethanol is usually visualized on US as a hyperechoic dense material that refills the anechoic empty chamber of the cyst. Since the needle may get occluded either during the fluid aspiration or the ethanol instillation phases, effective reaming may be achieved by repeatedly passing a stylet through the needle. In most instances, the volume of the ethanol injected corresponds to 50–70 % of the fluid volume drained from the cyst. However, the “right” ethanol amount should be chosen on individual basis, considering a number of factors: (a) the pattern of ethanol diffusion, (b) the degree of resistance applied to the tubing connection and the syringe, and (c) the subjective feedback from the patient. If the patient signals the sudden onset of intense pain or if there is resistance during ethanol injection, the administration of alcohol must be discontinued, and the position of the needle tip carefully checked.
After alcohol instillation, two alternative strategies have been used: (a) completely evacuate ethanol a short-time (3–10 min) after its instillation; (b) leave the cyst refilled with ethanol in order to prolong its sclerotic effect. Although the results obtained with these two modalities are similar [19] the latter strategy is probably preferred by most operators because is less cumbersome. Theoretically, the rationale for aspirating the ethanol-mixed fluid is to minimize the risk of ethanol leakage outside of the cyst into the surrounding tissue, an event that may lead to paraglandular fibrosis. Furthermore, there is a possible risk of post-surgical hypoparathyroidism and/or laryngeal nerve palsy in those patients previously submitted to PEI who are candidates for thyroid surgery [20].
35.2.1.4 Needle Extraction
At the end of the procedure, the needle is washed with saline solution to avoid minimal ethanol leakage to subcutaneous tissues and subsequent transient pain. Then, the needle is rapidly extracted. Firm compression and use of an ice pack for 5–10 min minimize bleeding. When the fluid is more viscous, the use of a larger bore needles (e.g., 18-gauge) should be considered for the drainage. For thyroid cysts with thick colloid material that cannot be easily aspirated, various authors have proposed modified EA techniques. The first strategy is a two-step approach: first, a small amount of ethanol is injected in an attempt to make the dense content more fluid. Then, 2–4 weeks later, a second aspiration with ethanol injection can be attempted [21, 22]. A second approach is the drainage of the dense colloid by means of a 16-gauge needle which is inserted into the nodule through a trans-isthmic approach and connected to a suction pump with a vacuum of 10–100 mmHg. If the content of the nodule cannot be aspirated, the 16-gauge needle is exchanged for an 8.5-French pigtail catheter. After more than 90 % of the viscous cystic content is aspirated, 99 % sterile ethanol is injected through the same needle or catheter. The amount of injected ethanol corresponds to 50 % of the aspirated fluid. After 10 min with the needle or catheter in place, the injected ethanol is removed completely [23]. This one-step EA method for viscous thyroid cysts is reported to produce volume reductions at 1 and 6 months of 78.4 % ± 14.4 % and 93.6 % ± 6.8 % respectively. Recently, a third option for the treatment of viscous cysts has been proposed utilizing a newly developed open-window needle that could efficiently aspirate dense gelatinous material from thyroid cysts [24].
35.2.2 Clinical Outcomes
A recently published Cochrane review concluded that, EA for cystic thyroid nodules results in a significant volume decrease, defined as a ≥50 % reduction, and improves pressure symptoms and cosmetic complaints with the only drawback of light pain after the procedure [25]. EA is considered the first-line treatment for thyroid cystic lesions [14, 15]. “Pure” thyroid cysts are exceedingly rare (<1 % of all thyroid nodules), and the majority of cystic thyroid lesions, about the 25–30 % of all thyroid nodules , correspond to mixed cystic solid lesions [26, 27] composed of an inhomogeneous fluid content of colloid, blood, and cell debris. Hemorrhagic cysts typically present as a suddenly visible neck lump which causes tenderness and pain, and is occasionally associated with hoarseness and dysphagia. Although these lesions may completely recede spontaneously or after fine-needle aspiration, they are quite likely to recur [28–31]. Studies comparing results of EA to simple fluid aspiration indicate that ethanol sclerotherapy offers a twofold higher chance of a long-term improvement with a 83 and 44 % of subjects achieving a significant reduction of the lesion volume after EA and aspiration alone, respectively [25, 32, 33]. Most studies report a low (<15–20 %) recurrence rate of thyroid cysts after ethanol sclerotherapy and recurrence rates of 3.4 % and 6.5 % at 5 years and 10 years, respectively [34, 35].
Interestingly, in spite of technical variations of the EA procedure, the results obtained across different studies report similar success rates, ranging from about 70 to 95 %, with a mean volume decrease between 65 and 90 % (Figs. 35.3 and 35.4) [32–37]. Resolution of local symptoms and cosmetic complains occurs in about 75–95 % of patients submitted to EA, although the quantification of symptom reduction is limited by the absence of standardized measurement tools. In a recent study, subjective symptoms were rated on a 10-cm visual analog scale [38]. Ethanol sclerotherapy was associated with a significant reduction of both symptom and cosmetic scores from 3.92 ± 1.54 to 0.39 ± 0.69 and from 3.31 ± 0.90 to 1.17 ± 0.56, respectively [38].



Fig. 35.3
A 6 mL right thyroid cyst before (a transverse view, b longitudinal view) and 2 weeks after (c transverse view, d longitudinal view) ethanol ablation . The thyroid cyst shrunk to 3 mL (50 % volume reduction)

Fig. 35.4
Cystic thyroid nodule before (a) and 18 months after PEI (b). The thyroid cyst volume has been reduced from 9 to 0.06 mL (99.3 % volume reduction) with disappearance of patient’s compressive and aesthetic problems
In another recently published series, a non-validated questionnaire including ten items related to goiter symptoms (visible enlargement, pressure in the neck, pain, difficulty in swallowing, shortness of breath and hoarseness, etc.) was created. Severity of each symptom was scored between 1 and 5 and a total score was calculated. EA reduced symptoms score from 22 ± 8 to 13 ± 5 (p < 0.05) [39].
The efficacy of EA depends on a number of factors, e.g., the initial volume of the lesion, the amount of ethanol instilled, the proportion of the solid component of the mixed cystic solid lesion. The efficacy of EA in exceedingly large (e.g., >40–50 ml) cysts has long been debated [32, 40] but a negative prognostic role of a higher initial cystic volume on the final outcome has not yet been clearly demonstrated [20, 41, 42]. The type of the nodule structure is a more likely determinant of the final result. While EA is commonly used for treating “pure” cystic thyroid nodules (liquid component >90 %) as well as “mixed” nodules with a predominant (50–90 %) fluid component, the latter ones usually achieve a maximum volume reduction of 60–65 %, less than the 90–95 % reduction achieved with “pure” cysts [43–48]. In “pure” cysts and in mixed nodules that are predominantly cystic, the final outcome is correlated to the volume of the fluid extracted, and to the amount of the instilled ethanol [20, 36]. Another, less predictable, obstacle to a successful PEI procedure is the finding of a viscous and dense cystic content [20, 36, 38]. A recent study based on 62 patients indicates that complete aspiration of the cystic content is the most important factor for achieving a successful EA in benign cystic thyroid nodules, as incomplete aspiration may significantly reduce the concentration of the injected ethanol [45]. Ethanol sclerotherapy has also been adapted, with encouraging results, to the treatment of thyroglossal duct cysts (TDC), a congenital neck abnormality deriving from the embryonic thyroglossal duct structures, located along the midline, between the hyoid bone and the thyroid gland [49–53].
35.2.3 Side Effects
EA of thyroid cystic lesions is a safe procedure and no relevant side effects are usually recorded [25]; nevertheless, because of the potential risk of recurrent laryngeal nerve (RLN) injury, vigilance with needle tip visualization is always required throughout the procedure. Ethanol injection into the cystic cavity is not likely to cause any major increase of pressure within the lesion and the integrity of the fibrous capsule surrounding the cyst should be fully preserved, thus preventing ethanol leakage into the perinodular tissues. As mentioned in the paragraph describing EA technique, the sudden pain during the phase of ethanol injection may signal improper alcohol diffusion outside the lesion. There are no reported differences in the risk profile of either complete aspiration of the ethanol-mixed fluid or leaving the ethanol within the lesion [19]. In one study, dysphonia , persistent nerve paralysis, and paranodular fibrosis were correlated to the use of larger ethanol doses while the occurrence of thyrotoxicosis and hyperpyrexia were unrelated to the amount of the instilled ethanol [54]. Most patients complain of a mild to moderate pain typically lasting a few minutes after the needle extraction. Pain, which can irradiate to the mandible angle or to the ear and rarely to the shoulder or to the back, may due to the contact of the needle tip with the subcutaneous tissues . Rinsing the needle with saline before its extraction may partially alleviate the discomfort but does not completely prevent it.
35.3 Alcohol Ablation of Thyroid Solid Nodules
35.3.1 Autonomously Functioning Thyroid Nodules
In the 1990s, several papers had enthusiastically proposed EA for the treatment of autonomously functioning thyroid nodules (AFTNs ) as a reliable and cost-effective alternative to surgery and radioiodine [10, 55, 56]. An initial optimistic success rate of EA (about 90 %), defined as complete or partial restoration of normal thyroid function, was reported in over 400 patients with AFTNs in various studies from different institutions [10, 12, 13, 57, 58]. Improvement of thyroid functional data was associated with an impressive decrease (58.5–90 %) of the volume of the nodules [10, 12, 13, 57–59]. Patients presenting with pre-toxic nodules were far more likely to respond to ethanol sclerotherapy as compared to overtly hyperthyroid patients (83.4 % vs. 66.5 %) as shown by a multicentric Italian study [59]. Furthermore, better results were apparently obtained in smaller nodules although a variable range of cut-off volume values (15–40 ml) has been suggested by different authors [60–62]. Nodule composition is also a critical factor for EA outcome in AFTNs, with a >30 % fluid component associated with a long-term treatment success, unrelated to the degree of baseline thyroid dysfunction [35]. Importantly, although EA of cystic nodules is usually accomplished in 1–2 sessions, EA of AFTNs routinely requires a higher number of sessions.
Only a minority of the initial studies reported more than a 1-year follow-up. Subsequently, studies examining long-term effects of EA for AFTNs have demonstrated a 13–35 % recurrence rate for hyperthyroidism [63, 64]. This finding is not surprising because scintigraphic studies showed the persistence of autonomous function tissue in 30–50 % of cases performed ethanol sclerotherapy of AFTNs [65, 66].
The side effects of ethanol sclerotherapy for AFTNs also limit its use. RLN palsy has been reported in 0.7–3.9 % of patients, and although often transient, its complete resolution cannot be reliably predicted [58, 62, 64]. Other less common but serious adverse events reported after EA in AFTNs include hematomas, ipsilateral facial dysesthesia, jugular vein thrombosis, septic complications, worsening of thyrotoxicosis, and transient Horner’s syndrome [67]. In addition, the later occurrence of Graves’ disease in patients previously submitted to EA has been linked to possible induction of anti-thyroidal autoimmune response by the toxic action of ethanol in the thyroid tissue [68].
Controversy about long-term beneficial effects of EA, in conjunction with serious side effects induced by the leakage of alcohol outside the lesion, has resulted in the abandonment of EA as a treatment for AFTNs [14, 15, 25, 69, 70]. The role of EA is presently confined to selected cases not amenable to treatment by first-line therapies; for instance, a multimodal therapeutic approach combining EA and radioiodine administration has been proposed in selected cases of large AFTNs as an option to reduce the required amount of 131I [65].
35.3.2 “Cold” Thyroid Nodules
Decreased nodule size has been reported after EA for benign nonfunctioning solid thyroid nodules [71, 72]. In a randomized, prospective study of 50 patients, EA achieved better results as compared to l-thyroxine (LT4) administration with a 47 % vs. 9 % mean volume reduction [72], while in a retrospective series on about 200 nodules a 75.1 % ± 12.3 (mean ± SD) volume reduction was reported [40]. The response of solid “cold” nodules, however, is less impressive than for cysts. Furthermore, repeated treatments are required with more frequent adverse effects [36, 54]. Thus, EA is not recommended for the treatment of solid cold thyroid nodules [14, 15, 25, 69, 70]. Today other more effective US-guided interventional techniques (e.g., laser thermal ablation and radiofrequency ablation) are available.
35.4 Alcohol Ablation in Other Neck Lesions
35.4.1 Parathyroid Lesions
EA of parathyroid (PT) lesions has been mainly applied in two clinical settings: treatment of non functional, large parathyroid cysts [73–75] and sclerotherapy of enlarged, hyperfunctioning PT glands in patients with chronic renal failure and tertiary hyperparathyroidism [76–79]. Cystic lesions of parathyroid origin may correspond either to a PT adenoma featuring a large fluid component or to nonfunctional “true” parathyroid cyst [80, 81]. A cystic PT adenoma is usually associated with primary hyperparathyroidism and sonographically demonstrates typically a solid, markedly vascularized portion in addition to a cystic area. Instead, non functional cysts have a “pure” fluid structure comprised of a watery, colorless fluid aspirate [73, 74, 81]. “True” PT cysts, which derive from embryological remnants [82, 83] are not as rare as once believed. Their incidence is underestimated because PT cysts are a rare cause of neck swelling, due to their usual deep location, posterior to the thyroid gland. Usually, PT cysts are incidentally detected during a neck US performed for various reasons, and they may be easily confused with a cystic lesion of thyroid origin [84, 85]. US-guided FNA with parathyroid hormone (PTH) assay in the aspiration fluid is useful for a correct diagnosis as well as recognition that an aspirate clear colorless fluid is almost pathognomonic for parathyroidal as opposed to thyroidal origin [3, 86, 87]. Similar to thyroid cysts , PT cysts frequently recur after FNA; therefore, ethanol and other sclerosing agents (e.g., tetracycline) are an effective alternative to surgery (Fig. 35.5) [73, 75–77, 88]. Although no major complications have been reported in published series, precaution should be taken to avoid ethanol leakage out of the cyst, because of close proximity to the laryngeal nerve in the tracheoesophageal groove. Severe tertiary hyperparathyroidism can be safely controlled using EA in patients with chronic renal failure [76–79, 89, 90]. Ethanol sclerotherapy of PT adenomas causing primary hyperparathyroidism should be restricted to patients at high-surgical risk [90, 91].


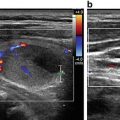
Fig. 35.5
A right, inferior, functional parathyroid cyst before (a) and after (b) ethanol ablation
35.4.2 Alcohol Ablation of Metastatic Lymph Nodes
Ethanol sclerotherapy of metastatic lymph nodes from differentiated thyroid cancer was first performed as a palliative procedure at the Mayo Clinic in the 1990s [92, 93]. Based upon results from the first published series of 14 patients treated from 1993 to 2000, Lewis and colleagues concluded that EA was a valuable tool for patients who were not candidates for further surgical or radioiodine therapy, and that the procedure was safe, less invasive than reoperation and could be repeated without major problems [93]. The application of EA to thyroid cancer recurrences has gained popularity with papers from international centers reporting their experience [94–100]. EA of metastatic lymph nodes is no longer confined to the palliative setting but also is now regarded as potentially curative in patients with localized neck persistence of disease [98]. In a recent review article, an 87.5 % success rate was reported in 168 patients treated with EA for lymph node metastasis in different institutions with a very low (1.2 %) risk of complications [101]. Four published series of patients with papillary thyroid cancer [95–98] report a 16.5–66.0 % rate of complete regression of the treated lesions and a remarkably low incidence of major complications. While EA of metastatic neck lymph nodes in the lateral neck compartments does not usually pose major concerns to the operators, apart from lesions strictly adjacent to large vessels , ethanol injections of lesions located in the central compartment may involve risk to the recurrent laryngeal nerves. The composition (solid vs. cystic) of the target lesion does not seem to influence the final outcome of the treatment. Furthermore, in the majority of patients treated by EA, subsequent progression of neck disease was not observed. Periodic measurement of serum thyroglobulin levels and US surveillance with color Doppler parameters are used to monitor the response to EA treatment. In conclusion, neck recurrences from thyroid cancer can profitably be treated by EA, after a careful risk-to-benefit balancing of the other therapeutic options. Future studies will help clarify advantages and limitations of ethanol sclerotherapy versus surgery and other interventional techniques in patients with neck recurrences from thyroid cancer [102–104].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree