LEARNING OBJECTIVES
1. List four broad categories of acute alveolar lung disease (ALD).
2. List five broad categories of chronic ALD.
3. Name three pulmonary–renal syndromes.
4. List five common causes of acute respiratory distress syndrome.
5. Suggest a specific diagnosis of ALD when supportive findings are present in the history or on the chest radiograph (e.g., broken femur and ALD in fat embolization syndrome, ALD and renal failure in pulmonary–renal syndrome, ALD treated with bronchoalveolar lavage in alveolar proteinosis).
Alveolar lung disease (ALD) refers to filling of the airspaces with fluid or other material (water, pus, blood, cells, or protein). The airspace filling can be partial, with some alveolar aeration remaining, or complete, producing densely opacified, nonaerated lung that obscures underlying bronchial and vascular markings. ALD producing dense airspace opacity is more easily distinguished from interstitial lung disease (ILD) than lesser degrees of alveolar filling. Abnormal “hazy” lung opacification that does not obscure underlying bronchovascular markings is referred to as ground-glass opacification and can represent ILD, ALD, or both. Compared with ILD, ALD tends to produce a homogeneous appearance of parenchymal opacification, with abnormal opacities appearing more confluent. ILD produces linear, reticular, nodular, or reticulonodular opacities. There can be, and often is, overlap in the radiologic appearances of ILD and ALD.
The different causes of ALD often cannot be distinguished on the basis of the radiologic distribution alone, but the clinical history, associated radiologic findings, and chronicity of the process can help to narrow the differential diagnosis. The processes to consider when an ALD pattern is seen can be divided into those that are acute and those that are chronic. Recurrence of an acute process can mimic a chronic process (as with recurrent pulmonary hemorrhage in pulmonary–renal syndromes [PRS]). In these cases, serial chest radiographs can help to show that the process is not caused by adenocarcinoma in situ (formerly bronchioloalveolar carcinoma [BAC]), lipoid pneumonia, or lymphoma, for example, because these processes are not expected to clear completely and then recur. All causes of acute ALD can resolve completely and subsequently recur, and therefore they should also be considered in the differential diagnosis of chronic ALD when serial chest radiographs or patient history suggests a chronic process with exacerbations and remissions. Although organizing pneumonia and eosinophilic pneumonia often present as ALD, they are discussed in Chapter 12 with other causes of peripheral lung disease.
ACUTE ALVEOLAR LUNG DISEASE
Pulmonary Edema
The four most common processes causing acute ALD are listed in Table 4.1. Pulmonary edema is the most common cause of ALD on chest radiographs. As mentioned in Chapter 3, on ILD, edema can be (a) hydrostatic (from cardiac failure, renal failure, or overhydration); (b) nonhydrostatic, owing to increased capillary permeability (in acute respiratory distress syndrome [ARDS] and fat embolization syndrome); or (c) inflammatory in etiology (as from chemical pneumonitis or eosinophilic pneumonia). Fat embolization syndrome occurs most commonly after traumatic fracture of long bones, which results in liberated marrow fat entering the pulmonary arterial circulation. Hydrolysis of fat, forming free fatty acids, leads to endothelial damage and increased capillary permeability 12 to 48 hours after trauma. This entity is further discussed in Chapter 8.
The radiologic distinction of pulmonary edema as cardiogenic or noncardiogenic in etiology is not always clear-cut (1). Radiographic signs of cardiogenic pulmonary edema include enlargement of the cardiac silhouette (which may be assumed as secondary to cardiomegaly in many cases but is not always distinguishable from pericardial effusion), pleural effusions, pulmonary vascular congestion and redistribution, and interstitial and alveolar opacities. Edema fluid spills into the interstitial spaces and progresses to filling of the airspaces. Often, the chest radiograph shows evidence of interstitial and airspace filling, although occasionally a predominantly interstitial pattern may be seen. Interstitial edema can result in blurring of the margins of blood vessels and hazy thickening of bronchial walls (peribronchial cuffing), thickening of fissures (subpleural edema), and edematous thickening of the interlobular septa (Kerley A and B lines). Subpleural pulmonary edema refers to fluid that accumulates in the loose connective tissue beneath the visceral pleura and is seen radiographically as a thickened fissure; this is sometimes difficult to distinguish from pleural effusion. Chest radiographs are highly sensitive for the diagnosis of pulmonary edema and can show edema in patients who have not yet developed symptoms; conversely, pulmonary edema may be visible radiographically for hours or even days after the hemodynamic factors have returned to normal (2).
Table 4.1 ACUTE ALVEOLAR LUNG DISEASE
“HEAP”
Hemorrhage
Edema
Alveolar proteinosis/Aspiration
Pneumonia (includes infectious, organizing, and eosinophilic pneumonias)
The distribution of airspace opacities in alveolar edema is usually patchy, bilateral, and widespread, and the opacities tend to coalesce. Air bronchograms may be evident, particularly when the edema is confluent. Often, alveolar accumulation of fluid in pulmonary edema is most pronounced centrally near the hila, resulting in a “bat’s wing” or “butterfly” configuration (Fig. 4.1). A clue to the diagnosis of pulmonary edema, instead of pneumonia, for example, is rapid change on radiographs taken over short time intervals (several hours); rapid clearing is particularly suggestive of the diagnosis (Fig. 4.2). Edema fluid can also change distribution or shift from one lung to the other as a result of the effect of gravity, as when a patient has been lying on one side.
ARDS is the result of increased pulmonary vascular permeability and develops in response to lung injury. The more common of the many lung insults leading to ARDS include sepsis, pneumonia, aspiration of gastric contents, circulatory shock, trauma, burns, and drug overdose. Often there are multiple overlapping inciting events. The clinical syndrome of ARDS is characterized by acute, severe, progressive respiratory distress, usually requiring mechanical ventilation; widespread pulmonary opacity on chest radiographs; hypoxia despite high inspired oxygen concentration; and decreased compliance of the lungs (“stiff lungs”). Damage to the alveolar capillary membrane leads to increased capillary permeability and leakage of proteinaceous fluid into the alveoli. Eventually, alveolar disruption and hemorrhage occur, surfactant is reduced, and the alveoli tend to collapse. The stages of ARDS are outlined in Table 4.2. The radiographic features may be delayed by up to 12 hours or more following the onset of clinical symptoms—an important difference from cardiogenic pulmonary edema, in which the chest radiograph is frequently abnormal before or coincident with the onset of symptoms. Findings on chest radiography include bilateral, widespread, patchy, ill-defined opacities resembling cardiogenic pulmonary edema, but without cardiomegaly, vascular redistribution, or pleural effusion (Fig. 4.3). Although the lungs appear diffusely involved on chest radiographs, computed tomographic (CT) scanning often shows a more patchy distribution with preservation of normal lung regions (3). If an endotracheal tube is not present on the chest radiograph, the diagnosis of ARDS is unlikely, except in the later stages of healing.
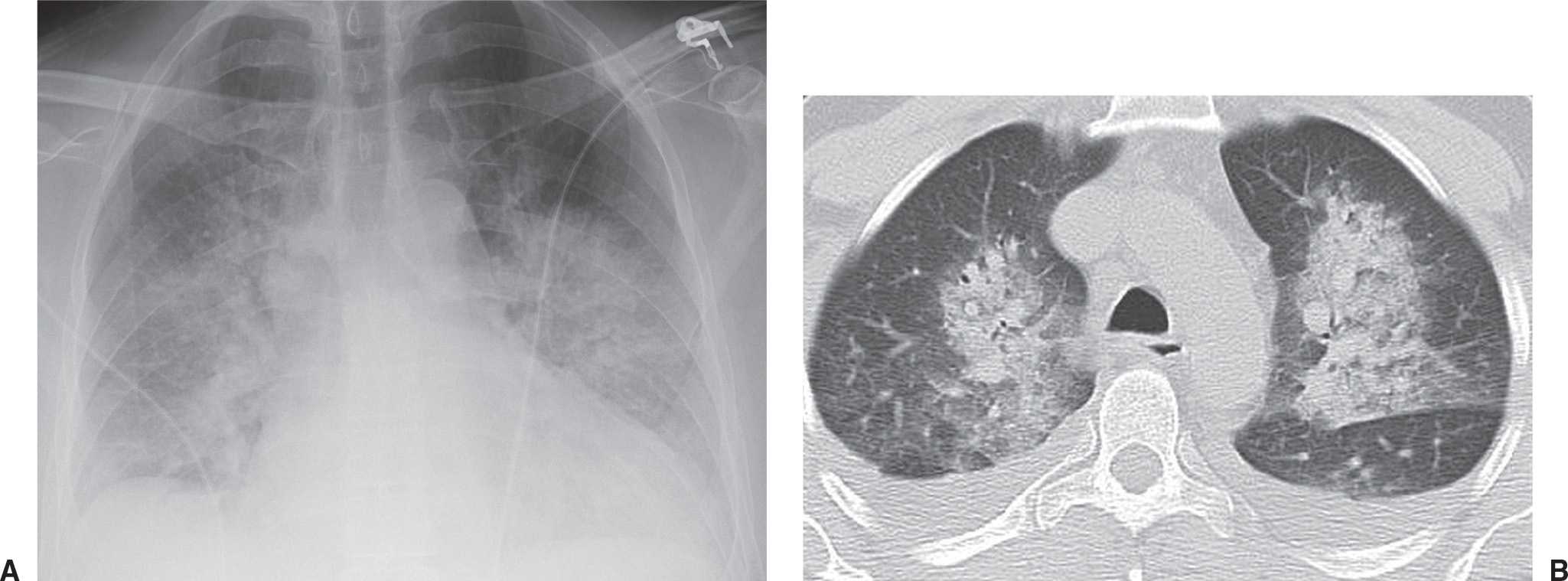
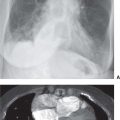
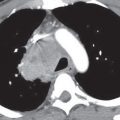
FIG. 4.1 • Pulmonary edema. This 79-year-old man suffered cardiac arrest and became pulseless and nonbreathing. A: Anteroposterior (AP) recumbent chest radiograph shows bilateral perihilar ALD. B: Computed tomographic (CT) scan shows bilateral central ALD and pleural effusions.
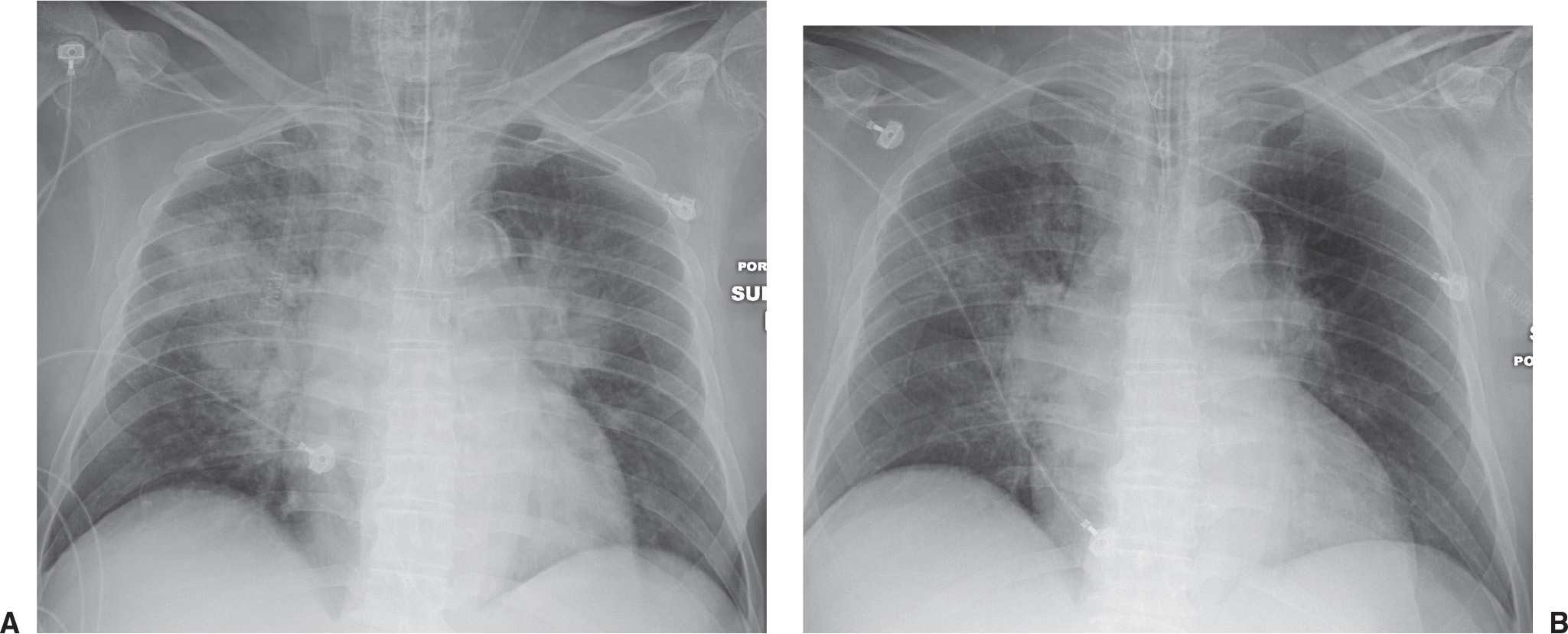
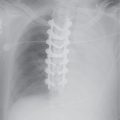
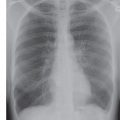
FIG. 4.2 • Pulmonary edema. This 47-year-old man had dilated cardiomyopathy and a left ventricular ejection fraction of 45%. A: Chest radiograph shows bilateral perihilar ALD in a “bat’s wing” configuration. B: Chest radiograph 2 days after (A) shows rapid improvement.
Patients with ARDS typically require mechanical ventilation, sometimes with high positive end-expiratory pressure because of stiff, noncompliant lungs. This predisposes to barotrauma, with rupture of alveolar walls and subsequent dissection of air into the perivascular bundle sheaths and interlobular septa, resulting in pulmonary interstitial emphysema. Discrete air-filled cysts, or “pneumatoceles,” may form in both central and subpleural locations (Fig. 4.4). These air collections can dissect into the mediastinum, causing pneumomediastinum, and can rupture into the pleural space, causing pneumothorax. The lung may be so stiff that it does not collapse easily, even when a pneumothorax is present. Air may dissect from the mediastinum into the neck and chest wall, retroperitoneum, or peritoneal cavity. The long-term outlook for survivors of ARDS is poorly documented. Mortality is related mainly to multiple organ failure rather than pulmonary dysfunction (4). One study of 109 survivors of ARDS showed that the survivors had persistent functional disability 1 year after discharge from intensive care. Most had extrapulmonary conditions, with muscle wasting and weakness being the most prominent (5). Chest radiographs may return to normal or show varying degrees of ILD, including pulmonary fibrosis.
Table 4.2 STAGES OF ACUTE RESPIRATORY DISTRESS SYNDROME
Stage 1 (first 24 hours): Capillary congestion and extensive microatelectasis with minimal fluid leakage. The chest radiograph may be normal, or it may show minimal interstitial edema or decreased lung volume.
Stage 2 (1 to 5 days): Fluid leakage and fibrin deposition and hyaline membranes develop. Alveolar consolidation by hemorrhagic fluid becomes extensive. The chest radiograph shows lung opacity (usually bilateral and symmetric), similar in appearance to cardiogenic pulmonary edema or pneumonia, which may start out patchy but rapidly coalesces.
Stage 3 (after 5 days): Alveolar cell proliferation, collagen deposition, and microvascular destruction. The chest radiograph shows a developing interstitial pattern that may result in honeycomb lung.
Reproduced with permission from Greene R. Adult respiratory distress syndrome: acute alveolar damage. Radiology. 1987;163:57–66.
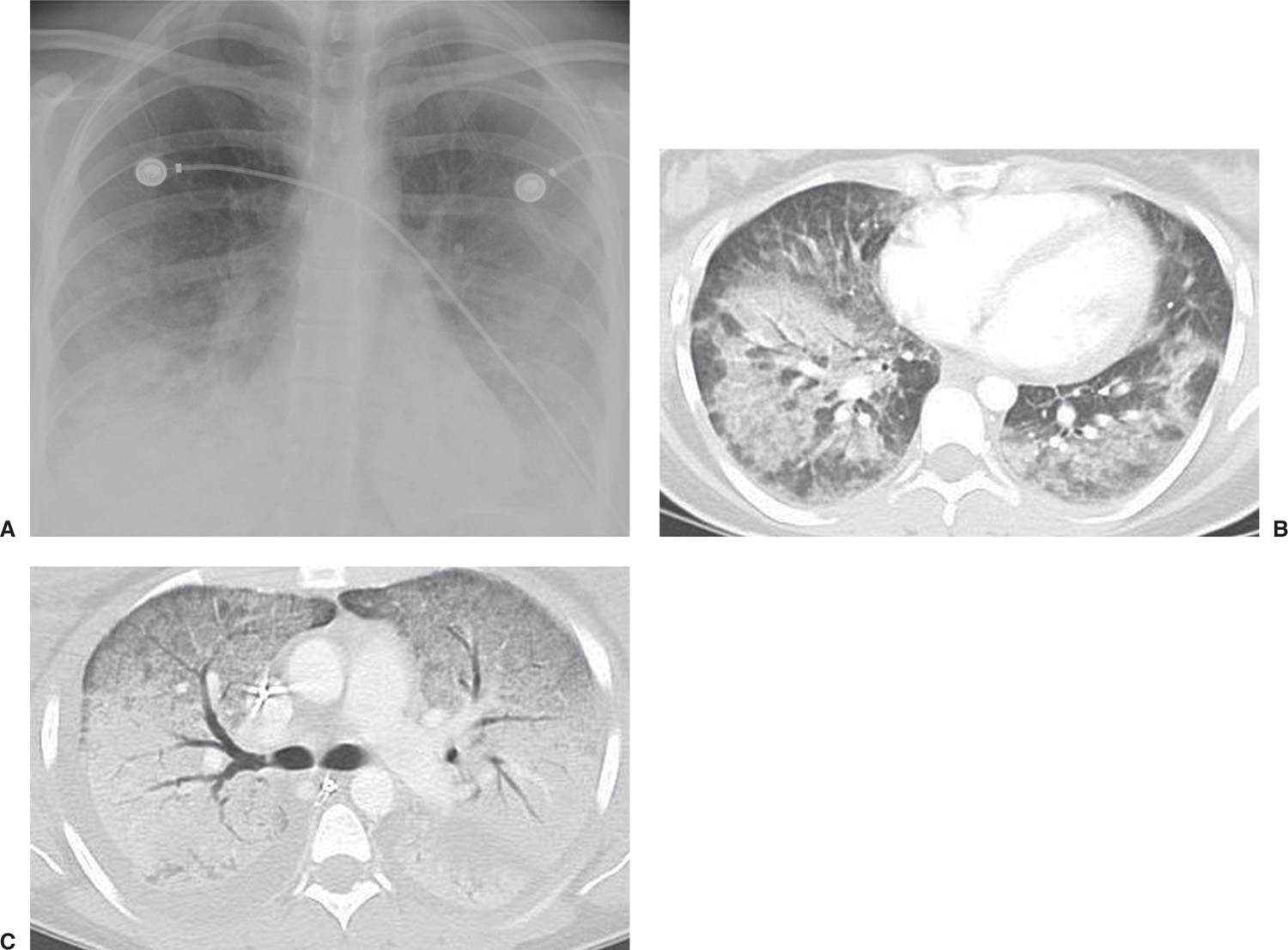
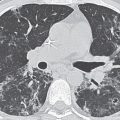
FIG. 4.3 • Acute respiratory distress syndrome (ARDS). This 27-year-old woman suffered toxic shock syndrome secondary to an infected intrauterine device. Posteroanterior (PA) chest radiograph (A) and CT scan (B) show bilateral ALD. C: CT scan 2 days after (B), after the patient developed ARDS, shows worsening bilateral ALD.

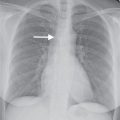
FIG. 4.4 • Acute respiratory distress syndrome. This 38-year-old man with a primary brain neoplasm developed severe respiratory distress requiring mechanical ventilation. A: AP recumbent chest radiograph shows bilateral ALD. An endotracheal tube is in place (arrowhead). Oval subpleural air collections represent pneumatoceles caused by barotrauma (arrows). A right subclavian pulmonary artery catheter was placed to measure pulmonary capillary wedge pressure. The pressure was low, consistent with noncardiogenic pulmonary edema related to ARDS. The tip of the catheter is projected over the left lower lobe pulmonary artery (curved arrow). B: CT shows bilateral ALD, multiple abnormal, rounded, and tubular air collections within the lung representing dilated airways (arrowheads), and a left pneumatocele (arrows).
Pulmonary Hemorrhage
Bleeding into the lung parenchyma occurs as the result of a variety of disorders (Table 4.3). A triad of features suggesting pulmonary hemorrhage is hemoptysis, anemia, and airspace opacities on chest radiography. Bleeding into the lung, however, does not always lead to hemoptysis (6). When bleeding into the lung is widespread, the pattern is referred to as diffuse pulmonary hemorrhage (DPH). The pulmonary features of all DPH syndromes are the same, and chest radiographs are generally not helpful in distinguishing among them. Lung opacities range from patchy airspace opacities to widespread confluent opacities with air bronchograms. The lung opacities show a perihilar or middle to lower lung predominance, and they tend to be more pronounced centrally, with sparing of the costophrenic angles and apices. In general, in cases of acute pulmonary hemorrhage (if there are no complicating factors), rapid clearing in 2 to 3 days can be expected. This can aid in narrowing the differential diagnosis when chest radiography shows diffuse ALD (7). When the airspace disease clears, interstitial opacities are often seen on chest radiography, as the result of by-products of blood breakdown being taken up by the septal lymphatics.
PRS are defined by the combination of diffuse alveolar hemorrhage and glomerulonephritis (8). The three most common causes of PRS presenting to the respiratory physician are antineutrophil cytoplasmic antibody (ANCA)-positive small-vessel vasculitis, anti–glomerular basement membrane (anti-GBM) disease (Goodpasture disease), and systemic lupus erythematosus (SLE). The ANCA-positive vasculitides describe three major systemic syndromes: granulomatosis with polyangiitis (GPA or Wegener disease), microscopic polyangiitis (MPA), and Churg–Strauss Syndrome (CSS).
Table 4.3 CAUSES OF PULMONARY HEMORRHAGE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree