Scan type
Radiotracer
Participants
Main findings
Reference
SPECT
123I-IMP
1 ALS-FTD 1 HC
Marked loss of frontotemporal uptake
Sawada et al. (1988)
17 ALS (1 FU after 9 months); 15 disease controls
Widespread uptake reduction, correlated with disease severity and duration
Ludolph et al. (1989)
1 ALS (16-month FU)
Extension of uptake reduction from PMC to frontal lobes over scan interval
Waragai et al. (1997)
99mTc-HMPAO
4 ALS-dementia
Reduced frontal lobe uptake (postmortem details included)
Neary et al. (1990)
14 ALS; 14 HC
8/14 prominently reduced frontal lobe uptake (3 with dementia, 1 with aphasia)
Waldemar et al. (1992)
18 ALS; 8 disease controls
Categorisation into four groups by reduced uptake: frontal + motor, mainly motor, just motor and no reductions, which then correlated with the spectrum of cognitive impairment
Abe et al. (1993)
19 ALS; 8 ALS-dementia; 29 FTD; 10 HC
Frontal and anterior temporal reduced uptake in all groups, greater in those with ALS-dementia and FTD
Talbot et al. (1995)
26 ALS; 26 HC
Reduced frontal uptake in those with cognitive impairment (postmortem details included)
Abe et al. (1997)
99mTc-ECD
35 ALS; 12 KD; 88 HC
Reduced frontal uptake in those with progressive bulbar palsy (correlated with bulbar functional scores); no change in KD patients compared with controls
Morimoto et al. (2012)
1 C9ORF72 fALS-FTD; 3 FTD
Hypoperfusion in regions overlapping with the resting-state functional MRI-derived ‘salience network’
Boeve et al. (2012)
PET – blood flow/metabolism
18FDG
12 ALS (5 FU); 3 poliomyelitis; 11 HC
Widespread and progressively reduced cerebral metabolism (not cerebellum) in those with clinical UMN signs
Dalakas et al. (1987)
3 PLS
Reduced uptake in pericentral cortex
Garnett et al. (1990)
18 ALS
Reduced glucose metabolism over entire cortex and frontal lobes. Some correlation with neuropsychological impairments
Ludolph et al. (1992)
3 PLS
Reduced uptake in pericentral cortex in 2/3 patients
Pringle et al. (1992)
7 ALS (3 FU); 1 PMA; 11 HC
No overall group difference. Progressive decrease in glucose metabolism in precentral gyrus but increase in middle frontal gyrus with progression
Hoffman et al. (1992)
3 PLS with Parkinsonian signs; control database
Precentral, prefrontal and cingulate uptake reductions
Mabuchi et al. (2004)
3 TARDBP fALS-FTD
Hypometabolism in frontal associative areas
Chio et al. (2010)
3 PLS
Focal hypometabolism in the precentral gyri – the ‘stripe sign’
Claassen et al. (2010)
32 ALS 22 HC
Increased metabolism in brainstem, amygdalae and cerebellum in all patients. Decreased metabolism in frontal and parietal regions in bulbar versus spinal onset
Cistaro et al. (2012)
6 ALS; 4 ALS-FTD
More extensive hypometabolism in ALS, including orbitofrontal cortex; relative preservation of anterolateral frontal metabolism in ALS patients, and perirolandic region in ALS-FTD
Renard et al. (2012)
1 ALS-dementia
FTD-like hypometabolism despite clinical Alzheimer-like dementia; C9ORF72 hexanucleotide expansion later identified
Adeli et al. (2014)
9 ALS;4 ALS-dementia; 13 HC
Hypoperfusion and hypometabolism in anterior cerebral hemispheres (and cerebellar hypoperfusion) in ALS-dementia
Tanaka et al. (1993)
15O2 and C15O2
12 ALS; 6 HC
Widespread impairment of activation, with expanded region of activation in response to movement task
Kew et al. (1993b)
10 ALS; 5 HC
Widespread reductions in blood flow in response to motor task, greatest in those with cognitive impairment (reduced verbal fluency)
Kew et al. (1993a)
12 ALS; 6 HC
Those with cognitive impairment (reduced verbal fluency) showed reduced frontal (insula and thalamic) reductions in rCBF in response to cognitive task
Abrahams et al. (1995)
H2 15 O
17 ALS; 17 HC
Widespread reductions in cortical binding
Lloyd et al. (2000)
PET – ligand
11C-flumazenil (GABAA receptor)
5 PLS
Regional cerebral blood flow reductions in fronto-opercular region, mainly precentral gyrus, extending to ventrolateral prefrontal region, and anterior cingulate cortex
Le Forestier et al. (2001)
24 ALS; 10 homozygous ‘D90’ SOD1 fALS; 2 presymptomatic homozygous ‘D90’ SOD1 carriers; 24 HC
Distinct less motor, more frontal reductions in binding in more slowly progressive ‘D90A’ (including two presymptomatics) compared with sporadic ALS patients
Turner et al. (2005b)
4 PLS; 24 ALS; 10 hom ‘D90’ SOD1 fALS 24 HC
Similar pattern of reduced binding in PLS and sporadic ALS patients. Preserved orbitofrontal binding in PLS versus both ALS groups
Turner et al. (2007a)
12 ALS
Reduced binding in right inferior frontal and superior temporal gyri plus insula with poorer verbal fluency. Reduced left middle frontal gyrus and left cuneus with poorer confrontation naming
Wicks et al. (2008)
10 ALS
Reduced binding in anterior cingulate gyrus related to writing errors
Yabe et al. (2012)
10 ALS 10 HC
Widespread binding, including CST, DLPFC, thalamus (linked to clinical UMN signs)
Turner et al. (2004)
11C-PK11195 (peripheral BZP receptor on activated microglia)
5 FTD 8 HC
Increased binding in frontotemporal regions
Cagnin et al. (2004)
1 PLS and 1 Mills’ syndrome patient with predominant right hemiparesis
Binding predominantly in left hemisphere
Turner et al. (2005a)
18 F-DPA-714 (TSPO ligand expressed on activated microglia)
10 ALS 10 HC
Significantly increased binding in primary motor, supplementary motor and temporal lobe cortices
Corcia et al. (2012)
11C-WAY100635 (5-HT1A receptor)
21 ALS (nondepressed); 19 HC
Marked frontotemporal reductions in binding
Turner et al. (2005c)
21 ALS (nondepressed); 11 homozygous (nondepressed) ‘D90A’ fALS; 19 HC
Proportionately increased regional binding in more slowly progressive familial ALS patients
Turner et al. (2007b)
4 FTD (nondepressed); 8 HC
Marked frontotemporal reductions in binding
Lanctot et al. (2007)
18 F-6-fluorodopa (presynaptic dopamine receptor)
12 ALS-parkinsonism-dementia complex of Guam; 7 HC
Intermediate presynaptic dopaminergic deficit in ALS-predominant patients (n = 4)
Snow et al. (1990)
16 ALS; 13 HC
Progressive decline in dopaminergic function with disease duration
Takahashi et al. (1993)
11C-raclopride (postsynaptic dopamine receptor)
1 FUS fALS; HC
Decreased putamenal binding (no clinical Parkinsonian features)
Kono et al. (2012)
29.2 Single-Photon Emission Computed Tomography
Early SPECT in ALS used the tracer N-isopropyl-p-123I-iodoamphetamine (123I-IMP). This revealed widespread cortical reductions, pronounced in those with longer disease duration (Ludolph et al. 1989) (Fig. 29.1). Soon afterwards 99mTc-HMPAO (hexamethylpropyleneamine oxime) SPECT revealed frontotemporal changes in four ALS cases with dementia (Neary et al. 1990), the same group proffering the now accepted concept of a continuum between ALS and FTD (Talbot et al. 1995). A comparative study involving patients with a slowly progressive LMN ‘mimic’ of ALS, Kennedy’s disease (KD, X-linked spinobulbar muscular atrophy), showed no equivalent decreases in cerebral blood flow (CBF) observed in the frontal lobes of the larger ALS patient group (when compared to healthy controls) (Morimoto et al. 2012).

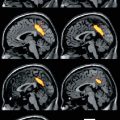
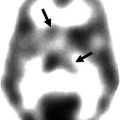
Fig. 29.1
123I-IMP SPECT in an ALS patient with dementia (a) compared to a healthy control (b). There is a clear loss of frontotemporal uptake in keeping with the understanding of extra-motor spread of pathology (Adapted from Sawada et al. (1988) with with kind permission of Springer Science+Business Media; superimposed boxes show quantitative sampling regions)
At least one third of cases with a family history of ALS have been linked to expansions of a hexanucleotide repeat within C9ORF72, and carriers within the same pedigree may manifest ‘pure’ (usually behavioural variant) FTD, ALS or mixed ALS-FTD (Majounie et al. 2012). A SPECT study in affected gene carriers showed a similar frontal hypometabolism in the single ALS-FTD subject to those with pure FTD, with the overall pattern showing overlap with the ‘salience network’ as defined by resting-state functional MRI (Boeve et al. 2012).
29.3 PET
29.3.1 Blood Flow and Metabolism
The initial application of PET to ALS was in studies of cerebral blood flow and metabolism, though these have been increasingly superseded by noninvasive functional MRI (Turner et al. 2012). Early and longitudinal studies with PET in ALS using 18F-fluorodeoxyglucose as a surrogate for cerebral metabolism (regional cerebral metabolic rate for glucose – rCMRGIc) confirmed widespread and progressive reductions in those ALS patients with clinical UMN signs (Dalakas et al. 1987). This was linked to neuropsychological deficits of a frontal lobe nature in ALS patients, particularly verbal fluency (Ludolph et al. 1992), in keeping with the predilection of ALS to cause a dysexecutive syndrome (Phukan et al. 2012). Alternative tracers H2 15 O and C15O2 were used to measure similar reductions in regional cerebral blood flow (rCBF) in ALS patients with dementia (Tanaka et al. 1993) and were also linked to abnormalities of verbal fluency in ALS patients without overt dementia (Kew et al. 1993a).
Some studies of patients with the UMN-only, consistently slowly progressive and related disorder primary lateral sclerosis (PLS) have demonstrated a strikingly focal, though inconsistent, hypometabolism of the precentral gyri (the ‘stripe sign’), in keeping with the frequent observation of atrophy in this region (Pringle et al. 1992; Claassen et al. 2010).
29.3.2 Ligand Studies
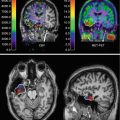
PET ligands have probed specific molecular pathways and provided unique in vivo insight into ALS pathogenesis (Fig. 29.2).
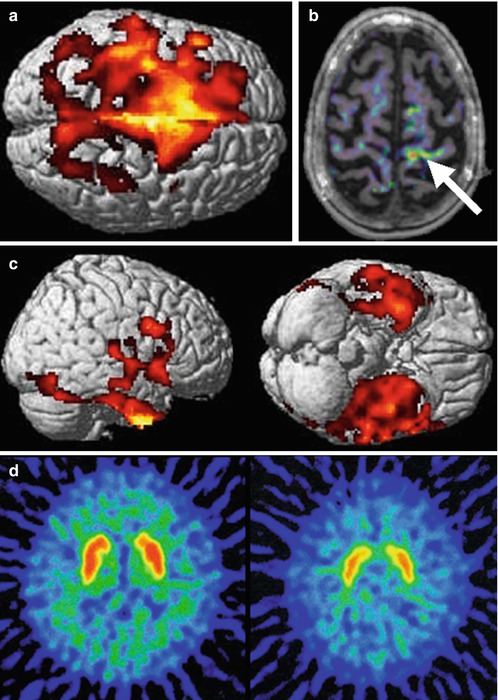

Fig. 29.2
Composite image from ligand PET studies in ALS, revealing different aspects of pathogenesis. A large area of motor and premotor cortex with reduced binding of the GABAA receptor ligand 11C-flumazenil in ALS patients compared with healthy controls (a) supporting a loss of interneuronal inhibitory influence. Evidence of activated microglia, as demonstrated by binding of the peripheral benzodiazepine receptor 11C-PK11195 (b) was localised to the left motor cortex (arrow) of a patient with weakness lateralised to the right-sided limbs. Right and inferior views of the brain show strikingly frontotemporal localisation of reduced binding of the serotonin 5-HT1A receptor ligand 11C-WAY100635 (c) in keeping with the pathological links between ALS and FTD. Reduced 11C-raclopride binding to the striatal postsynaptic dopamine D2 receptor (d) control subject on left, FUS fALS patient on the right) may reflect the occasional observation of Parkinsonian signs in those with ALS (Adapted from Turner et al. (2012) with permission of Future Medicine Ltd, and Kono et al. (2012), with kind permission of Springer Science+Business Media)
An Inhibitory Interneuronal Deficit
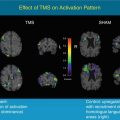
A variety of clinical and pathological evidence supports a potentially fundamental loss of cortical inhibitory influence with or without concomitant cortical hyperexcitability in the pathogenesis of ALS (Turner and Douaud 2012; Turner and Kiernan 2012). The ‘boundary shift’ in cortical activation seen in response to a joystick task led to the initial hypothesis of a loss of cortical inhibitory interneuronal circuits (Kew et al. 1993b) (Fig. 29.3). Widespread extra-motor cortical changes were then seen using the inhibitory neurotransmitter γ-aminobutyric acid (GABA)A receptor ligand 11C-flumazenil, providing further evidence for loss of inhibitory influences (Lloyd et al. 2000).
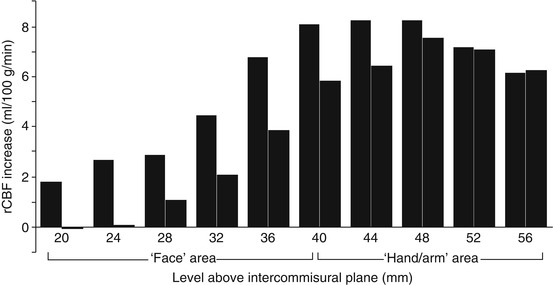

Fig. 29.3
Paired histogram showing mean regional CBF increases at ten points along the motor cortex in ALS (left column of each pair) and healthy controls (right column of each pair) in response to a motor task using hand-held joystick. The ALS patients demonstrated a ‘boundary shift’ in activation spreading to involve the face as well as hand motor areas. Loss of local inhibitory neuronal circuits was hypothesised (Adapted from Kew et al. (1993b), with permission of Oxford University Press)
Subsequent comparative 11C-flumazenil PET studies were carried out in familial cases associated with a rare recessive mutation in the superoxide dismutase-1 gene (SOD1) linked to a consistently slowly progressive form of ALS. These demonstrated relative preservation of tracer binding in motor regions, in patients matched for disability and UMN signs, supporting the view that preservation of inhibitory influence might have prognostic significance (Turner et al. 2005b).
Comparative studies in PLS revealed preservation of inhibitory influences frontally (Turner et al. 2007a), in keeping with the generally lower incidence of overt cognitive impairment in this group.
Neuroinflammation
A key role for neuroinflammatory mechanisms in the pathogenesis of ALS remains the subject of active research (Philips and Robberecht 2011; Evans et al. 2013). The PET ligand 11C-PK11195 binds to the peripheral benzodiazepine receptor expressed by activated microglia. A study in ALS patients provided in vivo evidence of widespread corticospinal tract and extra-motor microglial activation (Turner et al. 2004). A particularly tight correlation between thalamic 11C-PK11195 binding and UMN burden in this study may reflect the widespread connectivity of this structure and its disease-related disconnection in ALS. Cortical binding was notably lateralised within the hemisphere contralateral to the most affected body side in individuals with rare forms of motor neuron disease (Turner et al. 2005a). Immunohistochemistry studies have subsequently confirmed extensive microglial activation in the CST (Brettschneider et al. 2012). A new ligand for the translocation protein (TSPO) expressed by activated microglia – 18 F-DPA-714 – shows promise for future human ALS studies, with significantly increased binding in primary motor, supplementary motor and temporal lobe cortices, though with marked overlap between healthy control and patient distribution volume ratios (Corcia et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree