Fig. 1
Examples of the stimuli in the emotional faces task. Happy, angry or neutral faces were presented to the left or right of fixation, with their matched scrambled faces. Children responded with a left or right button press to indicate the side of the scrambled pattern, as quickly as possible
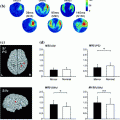
We conducted event-related beamforming analyses (Quraan and Cheyne 2010) on the MEG data in early time windows (60–200 ms). Happy and angry faces appeared to activate largely distinct brain regions (consistent with fMRI studies e.g., Kesler/West et al. 2001), and the patterns also differed with age group. In response to happy faces, we found that adults showed greater activity than adolescents, particularly at 100–140 ms, in left inferior frontal and inferior parietal lobule, as well as right middle temporal gyrus and superior parietal lobule (Fig. 2). At the same latency, adolescents showed greater activation in bilateral middle frontal regions. Adolescents showed greater activation to happy faces at 140–180 ms, with regions including bilateral inferior frontal, left superior frontal, and right superior temporal and middle frontal gyri. To angry faces, adults showed greater early (60–100 ms) activity in left inferior and right superior frontal regions while adolescents showed greater right middle frontal activation. Between 100–140 ms, adults showed greater right frontal and temporal activation to angry faces, whereas adolescents showed only greater left frontal and temporal activity (Fig. 2). This right hemisphere bias for adults, and left hemisphere bias for adolescents continued until 180 ms.
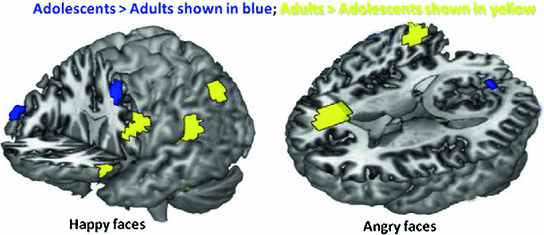

Fig. 2
Frontal and parietal activation to emotional faces differed between adults and adolescents between 100–140 ms—Adolescents > Adults shown in blue; Adults > Adolescents shown in yellow; happy (left) and angry (right). There was greater activation to the Happy than Angry faces in both age groups, and greater frontal activity to emotional faces in adults than in adolescents (in the left hemisphere for happy face, and the right for angry faces)
Early emotion-specific processing has been shown by Peyk et al. (2008) consistent with our findings of bilateral medial frontal activation in response to angry faces (140–180 ms) in adults, but no significant frontal activations to happy faces in this latency window. A number of studies have reported that the right hemisphere may be dominant for processing negative emotional stimuli (Killgore and Yurgelun-Todd 2007, Fournier et al. 2008); the current data suggest that if this is the case, this lateralisation of function is not complete even in the teenage years. Thus, these MEG data demonstrate that in adolescence the neural mechanisms underlying the development of rapid, implicit and emotion-specific processing are distinct from those seen in adults, suggesting that these processes are continuing to mature.
1.2 Inhibition Skills and Imaging Studies
Inhibition is a key process underlying social cognitive function, as inhibition of context-inappropriate behaviour is critical for successful social functioning. Behavioural studies of inhibition indicate reliable improvements from early childhood to adulthood (Luna et al. 2004), and the ability to produce sustained inhibitory control continues to improve through adolescence. Inhibitory control is supported by a widely-distributed circuitry in which frontal cortex plays a primary role (Rubia et al. 2007).
fMRI investigations in adults have identified a distributed network of brain areas involved in inhibition including striatal and thalamic structures, motor areas, anterior cingulate, parietal lobes and the inferior and dorsolateral frontal gyri (Rubia et al. 2001; Watanabe et al. 2002; Mostofsky and Simmonds 2008). The frontal cortex has been shown to play a major role in inhibition by studies that employed Go/No-go tasks where contrasts were made between the activations of No-go (successful response inhibition) to Go trials (response execution) (see review in (Dillon and Pizzagalli 2007)). Brain imaging findings in typical development, using Go/No-go tasks, vary extensively but have demonstrated a role for dorsolateral and inferior frontal regions in inhibition, although this involvement was not always reliably reported over childhood (e.g., Durston et al. 2002a; Tamm et al. 2004; Rubia et al. 2007).
Relatively few MEG studies have been conducted on inhibition and most included only a small number of sensors and/or subjects. We have tested adolescents and adults using MEG to determine the spatiotemporal brain dynamics of inhibitory control during the period of significant maturational changes over the teenage years. We used visual Go/No-go tasks that included a baseline condition with many more No-go than Go trials, allowing us to contrast only the No-go trials in the two runs, avoiding the confound of motor activity to the Go trials (Vidal et al. 2012). In this Go/No-go task, the Go stimuli were solid black shapes and No-go stimuli were the same shapes with a superimposed grey ‘X’ (Fig. 3). Similar to a recent ERP study (Bokura et al. 2001) we found right-lateralised frontal activity starting at 200 ms for the adults in the inhibition condition. Brain activations underlying inhibition in adolescents were slower, more superior and more bilateral in the frontal lobes compared to adults (Vidal et al. 2012). However, the low percentage (7 %) of Go trials in the control condition raised the possibility that the findings were influenced by an oddball effect. Thus, we ran a second study which also included two conditions, but the tasks contained inverse frequencies of Go to No-go trials for the experimental (67–33 %) and control (33–66 %) conditions to avoid an oddball confound and still allowed analysis of only trials pertinent to inhibition: the No-go trials (Vara et al. 2014). Only correct No-go trials from the two conditions were analysed.
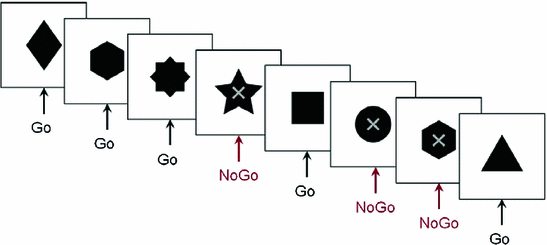

Fig. 3
Figure of Go/No-go protocol. An example of the stimuli in the Go/No-go tasks. Participants responded as quickly as possible to the stimuli except when there was a superimposed ‘X’
The spatiotemporal brain profiles involved in inhibition were examined in 15 adolescents and 15 adults with this Go/No-go task. Contrasting brain activation during No-go trials using vector event-related beamformer (Quraan and Cheyne 2010) showed recruitment of the right inferior frontal gyrus in adults (BA 45; 200–250 ms), but bilateral and delayed recruitment of similar locations in adolescents (BA 45/9; 250–300 ms) (Fig. 4). Activity near the hand motor region in the left hemisphere (BA 6) was present in both groups but persisted for a longer time in adults, suggesting that adolescents relinquished more rapidly the preparation to respond following the No-go stimuli. Adolescents also recruited the right temporal (BA 21) and inferior parietal (BA 40) regions during inhibition, likely reflecting increased attention-related resources being recruited (Durston et al. 2002b, Hampshire et al. 2010) to perform at adult levels. The findings of both delayed frontal and additional cortical recruitment in the teenagers compared to adults (Vidal et al. (2012), Vara et al. 2014) underline the immaturity of the inhibitory network in adolescence. These studies also highlight the importance of MEG to determine the temporal and spatial changes in brain processes related to the late maturation of inhibitory control over adolescence and into adulthood.
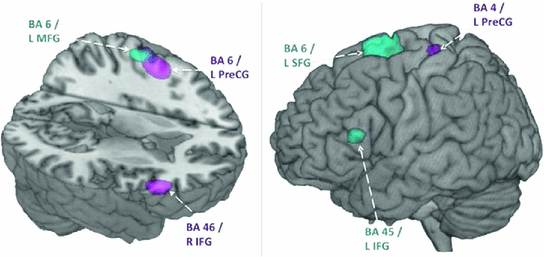

Fig. 4
Early activation in inhibition condition in adults and adolescents. Within group activations overlaid on brain images for 200–250 ms (left) and 250–300 ms (right). Inhibition condition > baseline condition, in adults shown in magenta (p < 0.005, uncorrected). Inhibition condition > baseline condition in adolescents shown in blue (p < 0.005, uncorrected). Note the early right IFG activation in adults, but the later left IFG in the teenagers. L Left, R Right, MFG Middle Frontal Gyrus, SFG Superior Frontal Gyrus, IFG Inferior Frontal Gyrus, PreCG, Precentral Gyrus
In summary, the frontal lobes are critical for cognitive processes underlying social as well as other executive functions; disturbances in their development have long-term, profound consequences. As the development of the frontal lobes continues into adulthood, their functions are the most susceptible to developmental disturbances; they are also the most amenable to modification with interventions, making the investigation of abilities dependent on the frontal lobes of considerable importance in atypical development.
2 MEG Studies of Executive Functions in Autism Spectrum Disorder (ASD)
Autism is described as a disorder encompassing abnormal social reciprocity, abnormal language use and an intense desire for sameness. While current definitions of ASD encompass varying degrees of difficulties in these three spheres, impairments in social interaction are the most striking feature which even affects individuals with high communication and cognitive functioning abilities (Frith 2004).
Despite considerable evidence of abnormalities of brain development in ASD, there is little consensus on how these findings lead to clinical and behavioural manifestations of ASD. Some findings suggest that some young children with ASD have a 5–10 % abnormal enlargement in total brain volume (Sparks et al. 2002, Courchesne 2004, Hazlett et al. 2005); the most reliable increases are reported in the frontal lobes, particularly in dorsolateral and medial frontal cortices (Carper and Courchesne 2005), which are areas strongly implicated in social cognitive function (Lewis et al. 2011, Telzer et al. 2011). Our own work with children 6–14 years of age found a trend for decreasing grey matter in typical children, but increasing grey matter in children with ASD (Mak-Fan et al. 2012). ASD children also show reduced measures of white matter integrity as assessed with DTI that are particularly marked in the long-range fibres and areas linked to social cognition (Cheng et al. 2010; Shukla et al. 2011; Mak-Fan et al. 2013).
2.1 Deficits in Social Cognition as Assessed with Emotional Faces in ASD
Face processing is central to much of social cognition. The behavioural literature has long reported face processing dysfunction in ASD; people with ASD exhibit poor eye contact (Hobson and Lee 1998) and look less at others’ faces (Langdell 1978). Many of the cognitive neuroimaging studies in ASD have focused on face processing, frequently finding atypical activation patterns in the fusiform gyri and/or amygdalae (Pierce et al. 2001; Amaral et al. 2003). In an ERP study we found that early responses to emotional faces were delayed and smaller in children with ASD (Batty et al. 2011), emphasising the need for the use of neuroimaging techniques with high temporal and spatial resolution, such as MEG, to investigate these issues. Face affect protocols in ASD participants have reported reduced activation in the medial prefrontal and STS regions (Pelphrey et al. 2007; Wang et al. 2007), key areas of the social brain network.
Emotional faces are arguably the most critical visual emotional stimuli and the ability to perceive, recognize, and interpret emotions is central to social interaction and communication. As impaired social interaction is one of the hallmarks of ASD, studies on the neural and cognitive mechanisms underlying emotional face processing in ASD are critical. Using an implicit emotional face processing task while acquiring MEG data (as detailed above), we examined spatiotemporal differences in neural activation during angry and happy emotional face processing in adolescents with and without ASD. The study included 12–15 year-olds, 14 in each group, with the controls being age- and sex-matched with the ASD teenagers. Both groups completed the Affect Recognition subtest of the NEPSY-II (Korkman et al. 2007); the scores on this test and response latencies on the emotional face task during MEG acquisition did not differ between groups. This argues that group differences found in neural activity on the emotional face task were not due to differences in perceived task difficulty. We focused on the MEG responses in the frontal lobes.
Early significant between-group differences to happy faces occurred at 80–120 ms, during which adolescents with ASD exhibited greater right superior and inferior frontal activation relative to controls. In contrast, controls showed greater superior temporal activation, relative to the ASD group, which is consistent with earlier findings of reduced superior temporal activation in individuals with ASD to faces (Pierce et al. 2001) and during social processing (Castelli et al. 2002) in a task that involved attributing mental states to geometric objects.
In response to the angry faces from 160–240 ms, we found greater left middle temporal activation in adolescents with ASD, relative to controls, consistent with the results of an emotional face matching task (Wang et al. 2004). This was in contrast, however, to greater left middle temporal activation seen in controls during explicit emotional face processing (Critchley et al. 2000), suggesting the involvement of different neural structures during explicit and implicit emotional face processing, also confirmed by our group (Bayle and Taylor 2010). In adolescents with ASD, greater activation occurred in the left BA 10 (superior frontal gyrus) while in typically developing controls, greater activation occurred in the right BA 10 (middle frontal gyrus). Our within-group analyses of orbitofrontal activation in controls to angry faces are consistent with evidence in the literature of the orbitofrontal cortex playing a role in anger processing (Blair et al. 1999; Luo et al. 2007). In controls, left orbitofrontal activation was first observed in BA10 from 120–160 ms, bilateral activation during 160–200 ms, which continued into 200–240 ms (right only). In contrast, adolescents with ASD show a delayed trend, with left BA10 activation starting at 200–240 ms, then bilateral activation from 240–280 ms. An atypical pattern of orbitofrontal activity to angry faces was seen in the adolescents with ASD compared to the controls; it was temporally shifted and had a different lateralization pattern Furthermore, between 200–240 ms, between-group comparisons showed greater left orbitofrontal activation in adolescents with ASD while controls showed greater right orbitofrontal activation. As left and right hemisphere advantages have been indicated for holistic and featural face processing, respectively, these findings are consistent with the notion that individuals with ASD utilise alternative face processing strategies, endorsing feature-based rather than holistic information processing (Hillger and Koenig 1991).
Of particular interest was the finding of similar MEG activity during happy, but not angry, face processing, suggesting more typical processing of happy faces in individuals with ASD. In response to angry faces, we found temporally delayed and contralateral activation of the orbitofrontal cortex, an area that has been implicated in anger processing. These data are also consistent with behavioural studies that show high functioning ASD participants perform comparably to controls on tasks involving happy faces, but have the most difficulty with angry faces (Kuusikko et al. 2009; Rump et al. 2009). Furthermore, our data suggest different emotional face processing strategies in individuals with ASD, who may use more local, feature-based processing strategies compared to typically developing individuals, who endorse more holistic strategies when processing emotional faces.
2.2 Difficulties with Inhibitory Control in ASD
Inhibitory control is often deficient in people with ASD, underlying in part their difficulties with emotional outbursts and inappropriate social behaviour. Given its protracted developmental course (Luna et al. 2010; Vidal et al. 2012), inhibition is an important area of investigation in teenagers with ASD, as they are adapting to increasing social demands. Luna et al. (2004) proposed that deficits in inhibitory capacities increase with age in autism, as the inhibitory demands increase in adolescence, a period of critical social development.
Some studies have investigated inhibition tasks in ASD. Adults with ASD show activation with notable differences in the patterns from that seen in controls, e.g., greater left frontal activity (Schmitz et al. 2006), reduced anterior cingulate activity (Kana et al. 2007), as well as in the timing of the brain activation within the frontal network. However, as inhibition is largely dependent upon the slowly maturing frontal lobes, the results from these adult studies cannot be used to generalise to a younger population. To date, there has been little research conducted on the period of brain maturation through adolescence in ASD, when adult levels of inhibition are being established in typically developing individuals which are critical to the establishment of normal social relationships.
To better understand the maturation of inhibition skills in ASD, we acquired MEG measures of brain activity during a Go/No-go task with adolescents and adults with ASD and age- and sex-matched controls (see Vara et al. 2014




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree