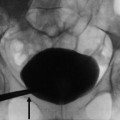
Fig. 1
Plain abdomen radiography demonstrating parenchymal calcifications (arrows) in a patient with medullary sponge kidney
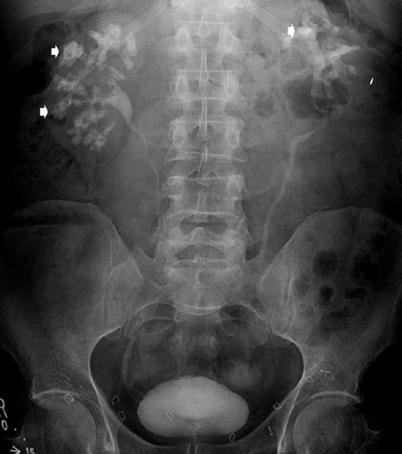
Fig. 2
Intravenous excretory urography demonstrating parenchymal calcifications (arrows) in a patient with medullary sponge kidney
2.2 Cortical Nephrocalcinosis
Cortical nephrocalcinosis is generally due to severe destructive cortical disease, often seen in patients with end-stage renal failure (Fig. 3). It is seen outlining the periphery of the kidney and along the columns of Bertin.
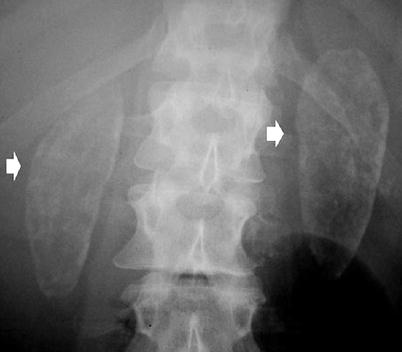

Fig. 3
Plain abdomen radiography demonstrating bilateral uniform cortical nephrocalcinosis (arrows)
Cortical nephrocalcinosis may be identified in many pathologic conditions such as acute tubular necrosis, chronic glomerulonephritis, Alport syndrome, prolonged hypercalcaemia and/or hypercalciuria involving many conditions which lead more frequently to nephrolithiasis (hyperparathyroidism, vitamin D intoxication, beryllium poisoning, systemic sarcoidosis, milk-alkali syndrome, hyperthyroidism, Addison disease, idiopathic hypercalcaemia of infancy, increased bone catabolism associated with myeloma, disseminated metastatic bone disease, leukaemia and immobilisation hyperparathyroidism) and chronic renal infections (tuberculosis, AIDS organisms, chronic pyelonephritis) and rejected renal transplants. Ethylene glycol (antifreeze) poisoning can cause marked ballooning and hydropic or vacuolar degeneration of the proximal convoluted tubules and actually corresponds to a form of acute tubular necrosis. Calcium oxalate crystals are usually found in the tubular lumen in such poisonings since ethylene glycol can lead to secondary hyperoxaluria.
Any form of chronic glomerulonephritis can give rise to nephrocalcinosis. The calcification in these cases is seen as fine, granular densities on standard X-rays. Acute cortical necrosis gives rise to patchy calcification, often related to episodes of hypovolaemic shock and eclampsia. Other described causes of cortical nephrocalcinosis include chronic pyelonephritis, Alport syndrome, renal transplants (Fig. 4), oxalosis, polycystic kidney disease and trauma.
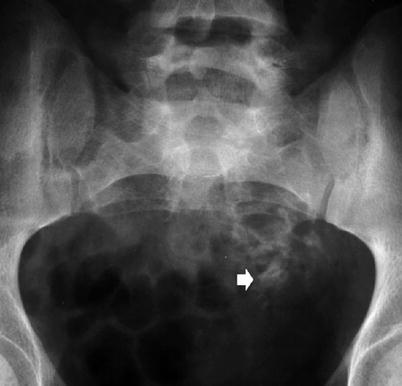

Fig. 4
Plain radiograph of the pelvis demonstrating cortical calcification (arrow) in a transplant kidney
2.3 Medullary Nephrocalcinosis
This form of nephrocalcinosis constitutes the most frequently identified type. Medullary nephrocalcinosis is characterised by diffuse calcium deposition within the renal medulla. Underlying causes include medullary sponge kidney, distal renal tubular acidosis, hyperparathyroidism, milk-alkali syndrome, hypervitaminosis D, chronic pyelonephritis, chronic glomerulonephritis, sarcoidosis, glycogen storage disease, Wilson’s disease, sickle cell anaemia, infections of the renal medulla, renal papillary necrosis and hyperuricemic (gout, Lesch-Nyhan syndrome) or hypokalaemic conditions (primary hyperaldosteronism, long-term furosemide therapy and Bartter’s syndrome). This appears radiologically as small clusters of calcification in the pyramids (Fig. 5). In some causes the pathophysiology is well understood, such as oxalosis and analgesic nephropathy (Figs. 6 and 7). In other cases, the pathophysiology is less well understood, with characteristic association with hypercalcaemia or hypercalciuria. Hyperparathyroidism and renal tubular acidosis are the most commonly recognised causes of medullary nephrocalcinosis along with medullary sponge kidney.
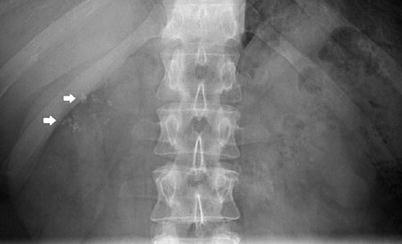
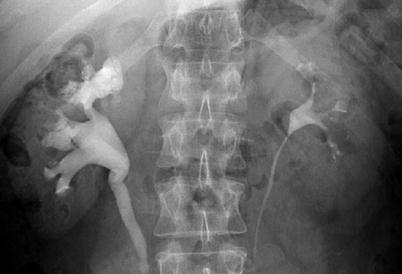
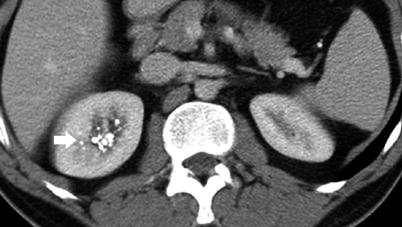

Fig. 5
Plain abdomen radiography demonstrating pyramidal calcification in the right upper pole (arrows)

Fig. 6
Intravenous excretory urography in a patient with analgesic nephropathy and a sloughed papilla causing hydronephrosis on the right kidney

Fig. 7
Contrast-enhanced computed tomography (CT) demonstrating pyramidal calcifications (arrow) in a patient with analgesic nephropathy
2.3.1 Abnormal Calcium Metabolism
All hypercalcaemic or hypercalciuric states can give rise to medullary nephrocalcinosis. The most common is primary hyperparathyroidism secondary to an adenoma, which causes increased mobilisation of calcium from bone. Hypervitaminosis D, which causes increased absorption of calcium, is a well recognised cause of hypercalcaemia and nephrocalcinosis. This may be accidental, self-induced or iatrogenic. The latter occurs despite normocalcaemia. Other causes include milk-alkali syndrome, sarcoidosis, immobilisation and hyperthyroidism.
2.3.2 Renal Tubular Diseases
Distal renal tubular acidosis is the second most common cause of medullary nephrocalcinosis. Most forms of distal renal tubular acidosis have a high incidence of nephrocalcinosis. The degree of nephrocalcinosis is more severe in these conditions than other causes. Calcium phosphate is believed to be deposited in the collecting ducts after precipitating from the urine. Fanconi’s syndrome, Wilson’s disease and amphotericin B toxicity are recognised common causes of secondary renal tubular acidosis. Proximal renal tubular acidosis does not manifest itself radiologically.
2.3.3 Medullary Sponge Kidney
The underlying aetiology in medullary sponge kidney is an anatomical defect of cystic dilatation of the distal collecting ducts. There is no underlying metabolic defect. Radiologically, this presents with dense papillary calcification, which has a characteristic appearance (Figs. 8, 9 and 10). The disease may manifest itself unilaterally or segmentally.
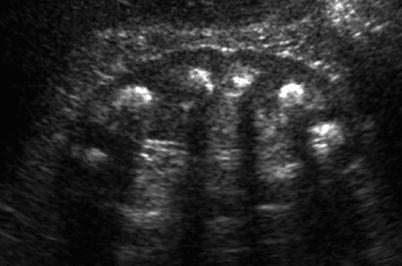
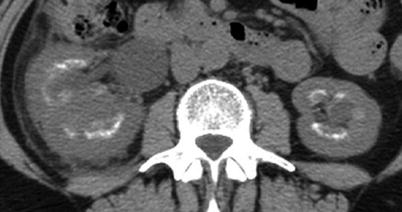
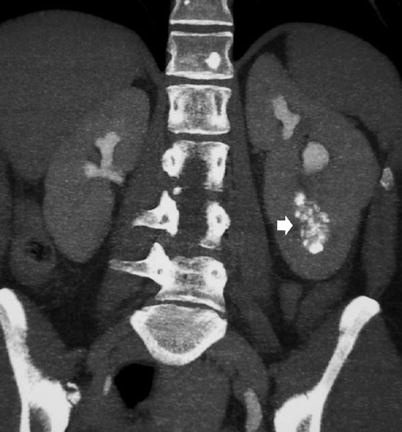

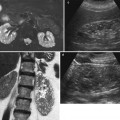
Fig. 8
Ultrasound (US) demonstrating extensive medullary calcifications, manifesting with focal hyperechogenicity with posterior acoustic shadowing, in a patient with medullary sponge kidney

Fig. 9
Unenhanced CT demonstrating extensive parenchymal calcification in a patient with medullary sponge kidney with perinephric stranding and an enlarged kidney

Fig. 10
CT urography. Coronal reformats. Renal sponge kidney with regional medullary nephrocalcinosis (arrow) (From the editor E. Quaia)
3 Nephrolithiasis
Nephrolithiasis is the description of the formation and passing of urinary tract calculi. The terms nephrolithiasis (refers to the kidney) and urolithiasis (refers to the ureter) indicate the presence of calcification(s) within the lumen of the collecting system, ureter and bladder. The commonest clinical manifestation is presentation with acute renal colic. Overall, world prevalence of nephrolithiasis is quoted at 2–3 % (Menon and Resnick 2002); however, this does vary around the world with different incidences quoted, depending on genetic background, race and climate. Calculus formation is related to an affluent lifestyle in developed countries. Peak incidence occurs in the fourth and fifth decade (Freeman and Sells 2008). Clinically, patients present with acute, severe loin pain when a renal calculus becomes impacted in the ureter causing obstruction, dysuria, strangury, haematuria and recurrent urinary tract infections. They can also present incidentally and chemically with dipstick haematuria, pyuria, sterile pyuria and proteinuria. However, calculi are often asymptomatic, identified as incidental findings on cross-sectional imaging. These incidental findings are, however, important as it can have important lifestyle implications including exclusion from certain occupations such as an airline pilot.
The clinical and metabolic presentation of stone disease can also change over time within various geographical regions. With careful clinical investigation and follow-up, it has been recognised that a cause for calculus formation can be elucidated in 97 % of cases (Rivers et al. 2000). Seventy percent of renal calculi contain calcium, and it is recognised that patients with this form of calculus disease will form further calculi within 7 years (Asplin et al. 1996) (Fig. 11).
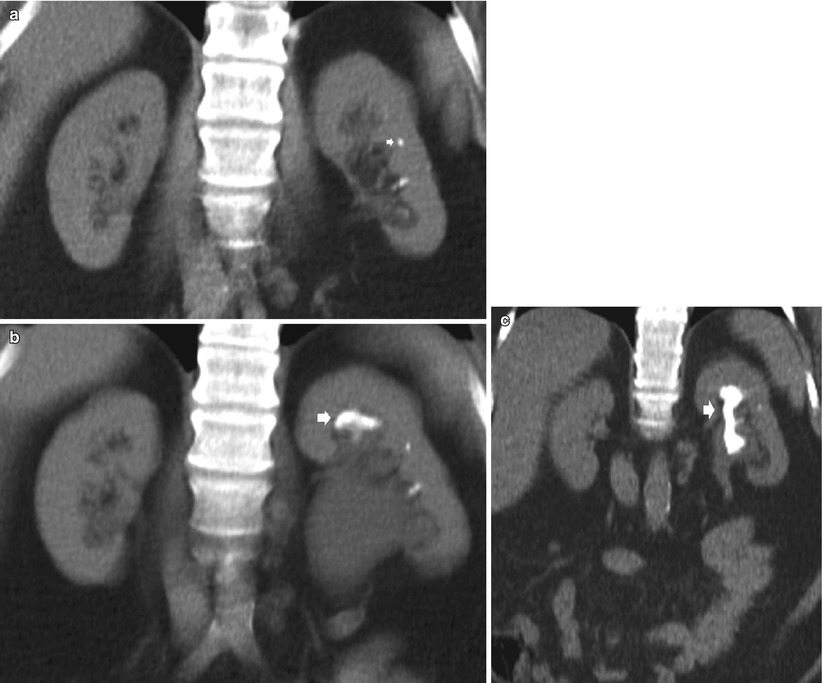

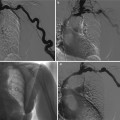
Fig. 11
A series of unenhanced CT coronal acquired over an 18-month period demonstrating initially a calculus (arrow) in the ureter causing hydronephrosis (a), then the progressive development of a staghorn calculus (arrows) (b–c)
The principal locations of renal calculi are calyceal, renal pelvis or ureteropelvic junction. If extracorporeal shock wave lithotripsy (ESWL) is considered, the size, location, and chemical composition of the urinary stones and the assessment of anatomic and functional anomalies in the upper urinary tract are of great importance in order to assess and ensure the smooth passage of the fragmented calculi (Saw et al. 2000; Boll et al. 2009). ESWL is an outpatient procedure, usually administered without anaesthesia. ESWL represents the most common mode of therapy for renal stones, and it is indicated for any type of stones, even though it works better for softer stones such as uric acid stones. A variety of stone characteristics, such as stone size, location and composition, may affect the success of ESWL. ESWL is indicated in the treatment of renal stones <2 cm in diameter.
Ureteric calculi are located distally in the ureter or ureterovesical junction. The American Urological Association published management guidelines for ureteric calculi (Segura et al. 1997). These guidelines recommend that patients whose calculi have a low probability of spontaneous passage (on the basis of their size and location) should be offered intervention. Newer semirigid, fibreoptic ureteroscopes can now be passed with minimal trauma and, in many cases, without dilatation. ESWL remains the first-line treatment for ureteric calculi, regardless of their site, with better results for radiopaque stones less than 1 cm in diameter. For proximal ureteral stones of 1 cm or larger, ESWL, ureteroscopy and percutaneous nephrolithotomy (PCNL) are all acceptable treatments (Teichman 2004). An endoscopic procedure (double J stent) can be inserted prior to ESWL in some cases, such as solitary kidney or large calculi. This decreases the number of complications. ESWL is contraindicated in pregnant patients, bleeding diathesis, uncontrolled hypertension, active urinary tract infections and obstruction distal to the stone. Complications include vascular injury, perirenal haematoma or subcapsular haematoma.
3.1 Incidence and Epidemiology
The risk of developing nephrolithiasis in the adult population varies in different parts of the world. The highest quoted incidence of nephrolithiasis is in Saudi Arabia at 20.1 %, with incidences of 1–5 % in Asia, 5–9 % in Europe and 12–13 % in Canada and North America (Ramello et al. 2000). Nephrolithiasis presents a peak incidence between 20 and 50 years of age and overall male-to-female ratio of 4:1. Epidemiological factors for stone-forming disease include race, sex, age and inherited and individual predisposing factors such as obesity. Environmental factors that influence formation include climate, socio-economic, diet and fluid intake.
3.1.1 Race and Sex
A higher incidence is quoted in males in the Caucasian population, which has been attributed to genetic make-up. However, it is noted that the incidence difference in Black Americans disappears when they adopt a Caucasian diet (Ramello et al. 2000). Conversely, the ratio is quoted as 0.5–0.7, respectively, male-to-female ratios in Black American and Hispanics (Michaels et al. 1994). The higher prevalence of nephrolithiasis in males has been attributed to the effect of androgens (Fan et al. 1999). Recent studies from the USA suggest that there is a changing gender prevalence of stone disease with a change from a 1.7:1 to −1.3:1 male-to-female ratio (Scales et al. 2007). This is speculated to be due to lifestyle factors and obesity.
3.1.2 Age
In idiopathic disease in males, a normal distribution of age is described with a peak incidence at 35 years. Females have a biphasic distribution peak, one at 30 years and the second at 55 years. This is thought to be secondary to calcium reabsorption secondary to the menopause.
3.1.3 Inherited Diseases
The incidence quoted for disease such as cystinuria (autosomal recessive) and hyperoxaluria is scant. Multiple predisposing factors have been identified including general factors (male gender), inherited conditions (polycystic kidney disease, renal tubular acidosis, hyperparathyroidism, cystinuria and hypercalciuria), medications (triamterene, sulphonamides, carbonic anhydrase inhibitors, indinavir, acetazolamide, corticosteroids), low urine volume, hypercalciuria (hyperparathyroidism, sarcoidosis), hyperoxaluria and hypocitraturia (distal renal tubular acidosis). The prevalence of cystinuria is estimated at 2 % in the general population.
3.1.4 Individual Predisposing Factors
Obesity is associated with an increased risk of renal calculi, especially in females with a BMI over 40. Hypertensive patients are more likely to suffer from renal calculus disease. Factors influencing this are complex and may be due to the antihypertensive therapy and other dietary factors.
3.1.5 Environmental Factors
Calculus disease is more prevalent in a temperate climate. This has been described secondary to decreased fluid intake combined with high plasma vitamin D serum levels secondary to sun exposure.
3.1.6 Socio-economic Factors
Calculus disease is seen as a disease of more affluent nations.
3.1.7 Diet
A high-protein diet has influence on calculus formation risk and calculus composition. Uric acid and calcium oxalate calculi are more prevalent in those with a diet high in animal proteins. The role of dairy products in the pathogenesis of renal calculus disease remains in debate.
3.2 Pathophysiology
Despite significant progress in the treatment of renal calculi, the degree of understanding of the pathogenesis of renal calculus disease has not paralleled this. It has been described that urinary supersaturation is necessary for clinical stone formation. This precipitates crystals to form in the urine. The crystals are believed to be retained in the tubules of the kidney. Several substances found in urine have been demonstrated to precipitate crystal formation, e.g. pyrophosphate, Tamm-Horsfall proteins and magnesium. Noncrystalline organic material, matrix, is then combined with crystal to form calculi. The mode that this occurs remains a source of ongoing research. Several theories have been proposed for pathogenesis. One such theory is crystal-induced renal injury secondary to hyperoxaluria, free particle hypothesis. This proposes that there is a rapid crystal growth in the tubular lumen resulting in crystals being trapped in the papillary collecting duct, resulting in stone formation. An intravascular phenomenon has been proposed with calculi forming as a result of this phenomenon in the vasa recta. Nanobacteria have been proposed as cytotoxic gram-negative bacteria are also implicated in other diseases such as atherosclerosis and periodontal disease. Urinary stasis secondary to anatomic abnormalities is also an identified cause, e.g. hydronephrosis, ureteropelvic junction obstruction and horseshoe kidney. Randall’s plaques were described over 60 years ago; this suggested that calculi formed around calcium salt deposits in the tip of the renal papilla.
3.3 Types of Calculi
3.3.1 Calcium Calculi
Calcium oxalate stones account for 60 % of all renal stones. This is followed by calcium phosphate types (hydroxy-apatite 20 % and brushite 2 %). Hypercalcaemia is the most important pathophysiological risk factor. This can be divided into three categories:
1.
Absorptive hypercalciuria due to increased stone absorption of calcium
2.
Renal hypercalcaemia due to renal tubular resorption of calcium, which precipitates increasing PTH secretion due to low serum calcium
3.
Resorptive hypercalcaemia associated with primary hyperparathyroidism
Other causes of hypercalciuria include malignancy, sarcoidosis, hyperparathyroidism and vitamin D toxicity. Hyperoxaluria, hyperuricosuria and gout can also give rise to calcium oxalate calculi.
Calcium oxalate calculi are dense and therefore relatively well identified on abdominal radiographs.
3.3.2 Cystine Stones
Cystinuria is an autosomal recessive disease that results in a defect in the renal tubular absorption of amino acids, which in turn results in cystinuria. These calculi are only identified on abdominal radiographs if they contain calcium.
3.3.3 Struvite or Infective Calculi
Struvite calculi are composed of magnesium and ammonium phosphate and account for between 7 and 13 % of all renal calculi in the western world (Gleeson and Griffith 1993). They are formed secondary to infection by urease-producing bacteria, most commonly Proteus mirabilis. Struvite does not crystallise in the urine if the pH is less than 7.19. As urinary tract infections are more frequent in women, struvite calculi are seen in females more frequently than males (2:1). Pure struvite calculi are relatively rare; the most common manifestation of struvite calculi is triple stones, calcium-magnesium-ammonium phosphate stones. This is the most common composition of staghorn calculi (Figs. 11, 12 and 13) and is clearly seen on abdominal radiographs. Pure struvite stones are radiolucent.

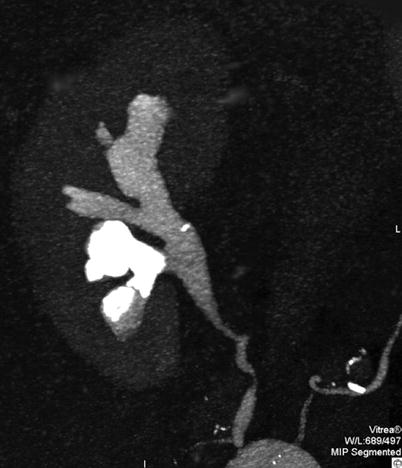

Fig. 12
Greyscale ultrasound. Longitudinal scan. Staghorn renal stone with multiple hyperechogenicities with posterior acoustic shadowing in the renal pelvis

Fig. 13
CT urography. Coronal reformat. Staghorn stone in the lower calyceal group of the right kidney
3.3.4 Uric Acid Calculi
The incidence of uric acid stones varies from 40 % in Israel to 10 % in the USA. They are associated with obesity and type II diabetes. Three factors contributing to uric acid stones are hyperuricosuria, acidic urine and low urinary volume. Low urine pH is often related to medication and also associated with chronic diarrhoea and an ileostomy. A low urinary volume, less than 2 L/day, is considered to predispose to calculus formation. Hyperuricosuria is related to several inherited metabolic disorders such as Lesch-Nyhan syndrome and glycogen storage disease types I, III, V and VII. Uric acid stones are radiolucent on abdominal radiographs. They, however, have sufficient density to be demonstrated in CT and cast an acoustic shadow on ultrasound (US).
3.3.5 Xanthine Calculi
Xanthine calculi are seen in patients with hereditary xanthuria and those undergoing treatment with allopurinol. They are rare calculi with a density similar to that of uric acid calculi and have similar imaging characteristics.
3.3.6 Matrix Calculi
Like struvite calculi, matrix calculi are typically seen in patients with underlying proteus infections, as well as other infections such as Escherichia coli and Candida albicans. Their composition is mainly of coagulated mucoids with very little calcium component. They are radiolucent on abdominal X-rays.
3.3.7 Indinavir Calculi
Indinavir sulphate is a drug used widely to treat HIV infections. It is a protease inhibitor, and during clinical trials, 4 % of patients receiving the therapy developed calculi. Indinavir crystals are precipitated in the urine forming calculi. These are radiolucent on both plain radiographs and CT (Schwartz et al. 1999). Calcium oxalate and phosphate precipitate within the indinavir crystals, and with time, the density all of the calculi will increase enough to become visible on CT and abdominal radiographs. Contrast-enhanced CT in the delayed phase is valuable in the diagnosis of non-calcified indinavir calculi (Blake et al. 1998).
3.3.8 Oxalate Calculi
Oxaluria may be primary or secondary relating to underlying disorders such as inflammatory bowel disease and short, small bowel syndrome. This occurs due to fat malabsorption leading to saponification of calcium, leaving oxalate unbound, which is then absorbed in the mucosa of the colon.
Patients with coeliac disease as well as Crohn’s disease have increased absorption of oxalate from the colon. Patients with inflammatory bowel disease are at increased risk of urolithiasis of all types, with a reported prevalence of 2–12 % (Mcleod and Churchill 1992). Increased consumption of leafy green vegetables that are high in oxalate can also give rise to hyperoxaluria. This results in nephrocalcinosis and progressive formation of calcium oxalate stones. The majority of patients with calcium oxalate stones may not have any detectable abnormality of oxalate metabolite or hyperoxaluria. Oxalate calculi are usually radio-opaque on abdominal radiographs.
4 Imaging
4.1 Background and Principles
The main aims of imaging in patients with acute flank pain that can be related to renal colic are the detection of calculi or the non-stone disease; detection of non-urinary disease related to flank pain; assessment of size, number and location of the stone(s); detection of complications; and evaluation of the contralateral kidney. Four imaging techniques are employed to diagnose renal colic are (1) plain abdominal radiography (kidney-ureter-bladder radiograph; KUB), (2) US, (3) intravenous urography and (4) helical CT.
Identifying the position of calculi anatomically will influence clinical management and intervention. Imaging should establish the physiological impact of the calculus, again influencing the ongoing management of renal disease. Abdominal radiography and intravenous urography were the main stay of imaging strategies until the end of the last century, along with US assessment. The advent of helical CT has seen these modalities largely superseded by a non-contrasted helical CT and excretory CT urography.
4.2 Abdominal Radiographs
The majority (90 %) of renal tract calculi are dense enough to be identified on standard KUB abdominal radiographs (Spirnak et al. 1990) (Figs. 14 and 15). Renal calculi are usually single, polymorphic and with homogeneous opacity. Phleboliths typically appear as multiple round or oval opacities with a central lucency and lateral to the inferior portion of the sacrum (Fig. 16). The sensitivity of the plain abdominal radiography in the detection of urinary calculi ranges from 44 to 77 %, while the specificity ranges from 80 to 87 % (Svedstrom et al. 1990; Haddad et al. 1992; Dalla Palma et al. 2001).
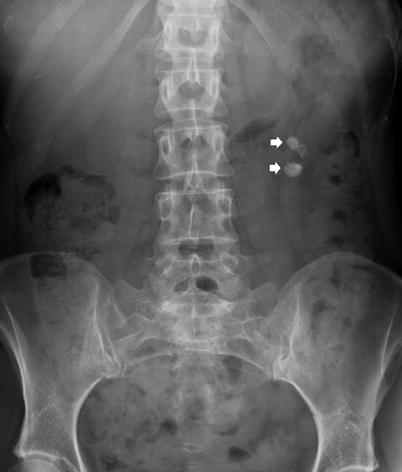
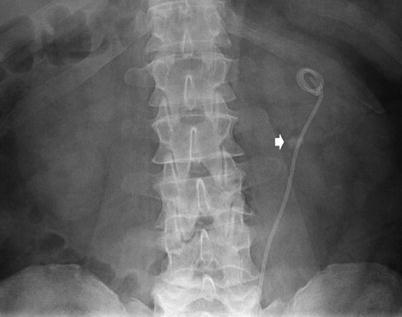


Fig. 14
Plain abdomen radiography demonstrating calculi (arrows) within calyces of the left kidney

Fig. 15
Plain abdomen radiography with ureteric stent in situ demonstrating a calculus (arrow) within the renal pelvis

Fig. 16
Plain abdomen radiography. Pelvic phleboliths appearing as multiple round or oval opacities with a central lucency (arrows), and lateral to the inferior portion of the sacrum
One of the main limitations of this technique is the presence of bowel gas and faecal material within the large bowel, which can obscure small calculi. The principal reasons for the limited visibility of renal stones on the plain radiography are bone overlapping and extra-urinary calcifications including vascular calcifications, calcified lymph nodes, focal zones of compact bone and phleboliths. Patient body habitus can also give rise to interpretation difficulties. In patients presenting with loin pain, the KUB radiograph is often the initial investigation; however, it has been argued that this investigation is not necessary in the investigation of acute renal colic if unenhanced CT is utilised (Kennish et al. 2008).
The KUB is an extremely valuable tool in the follow-up of known renal calculi, especially those undergoing treatment and intervention for calculus disease (Fig. 15).
4.3 Intravenous Excretory Urography
In the USA, this examination has largely been superseded by unenhanced helical CT. However, in Europe and other parts of the world, this technique continues to be utilised where access to CT scanning may be limited. Intravenous excretory urography is a time-consuming technique, especially in obstructed patients; adds the risks of iodinated contrast administration; and is not suggested in acute ureteral obstruction due to contrast-induced diuresis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree