ASA physical status 1—A normal healthy patient
ASA physical status 2—A patient with mild systemic disease
ASA physical status 3—A patient with severe systemic disease
ASA physical status 4—A patient with severe systemic disease that is a constant threat to life
ASA physical status 5—A moribund patient who is not expected to survive without the operation
ASA physical status 6—A declared brain-dead patient whose organs are being removed for donor purposes
Risk factors for cardiac complications have long been known. In 1977, Lee Goldman, M.D. attempted to stratify cardiac risk for patients undergoing non-cardiac surgery. He evaluated patients based on risk factors known as the Goldman Cardiac Risk Index or CRI and assigned each a point. Adding up the total points assesses the probability of risk for perioperative cardiac morbidity and mortality. Subsequent studies have shown that some surgical procedures carry minimal risk, while others have excessive risk which this index does not take into account. The American Cardiology College (ACC) and American Heart Association (AHA) have expanded this assessment to include risk associated with a particular surgical procedure as well as patient characteristics that influence perioperative cardiac morbidity and mortality [17–19] (Fig. 2.1 and Tables 2.2, 2.3, and 2.4).
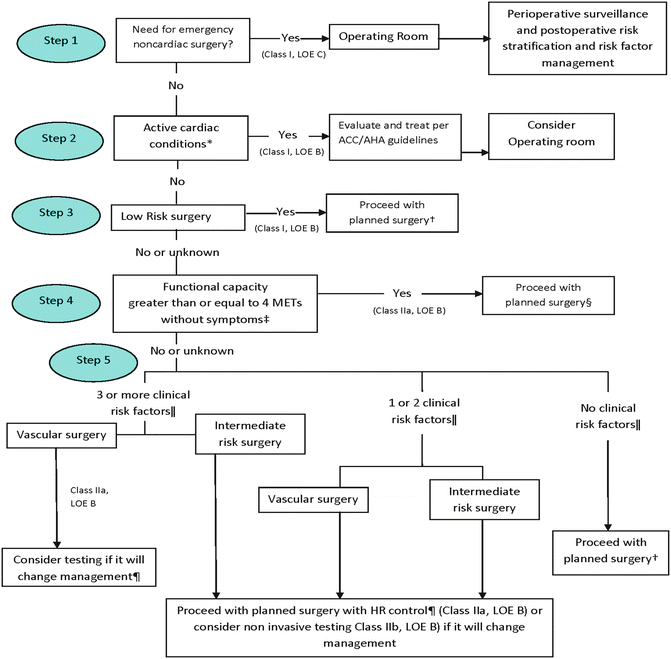

Fig. 2.1
Cardiac evaluation and care. ACC/AHA indicates American College of Cardiology and American Heart Association, HR heart rate, LOE level of evidence, and MET metabolic equivalent. From Fleisher et al. [19]. Reprinted with permission from Wolters Kluwer Health
1.History of ischemic heart disease |
2.History of congestive heart failure |
3.History of cerebrovascular disease (stroke or transient ischemic attack) |
4.History of diabetes requiring preoperative insulin use |
5.Chronic kidney disease (creatinine >2 mg/dL) |
6.Undergoing suprainguinal vascular, intraperitoneal, or intrathoracic surgery |
Table 2.3
Cardiac risk stratification for non-cardiac surgery
High (reported cardiac risk often greater than 5 %) |
• Emergent major operations, particularly in the elderly |
• Aortic and other major vascular surgery |
• Peripheral vascular surgery |
• Anticipated prolonged surgical procedures associated with large fluid shifts and/or blood loss |
Intermediate (reported cardiac risk generally less than 5 %) |
• Carotid endarterectomy |
• Head and neck surgery |
• Intraperitoneal and intrathoracic surgery |
• Orthopedic surgery |
• Prostate surgery |
Low (reported cardiac risk generally less than 1 %) |
• Endoscopic procedures |
• Superficial procedure |
• Cataract surgery |
• Breast surgery |
Table 2.4
Estimated energy requirements for various activities
Estimated energy | Activity |
|---|---|
1 MET | Self-care |
Eating, dressing, or using the toilet | |
Walking indoors and around the house | |
Walking one to two blocks on level ground at 2–3 mph | |
4 METs | Light housework (e.g., dusting, washing dishes) |
Climbing a flight of stairs or walking up a hill | |
Walking on level ground at 4 mph | |
Running a short distance | |
Heavy housework (e.g., scrubbing floors, moving heavy furniture) | |
Moderate recreational activities (e.g., golf, dancing, doubles tennis, throwing a baseball or football) | |
Greater than 10 METs | Strenuous sports (e.g., swimming, singles tennis, football, basketball, skiing) |
The approach to the patient’s cardiac assessment includes a detailed history of previous diagnoses of coronary artery disease (CAD), any prior cardiac interventions including previous cardiac testing, current therapies, symptoms associated with angina, or congestive heart failure and if the patient has a pacemaker and/or implantable cardioverter defibrillator [20]. It is important to identify any new or change in symptoms which may require a change in or initiation of new management that would be the cause of delay or cancellation of non-elective surgeries. Perioperative morbidity related to non-cardiac surgical procedures ranges from 1 to 5 % [20, 21].
Patient risk for CAD should be evaluated. Diabetics have a higher probability of CAD than nondiabetics. It is important in these patients to determine the length of disease and the degree of end-organ dysfunction as they have a higher association of silent myocardial ischemia and myocardial infarction. Diabetes has been shown to be an independent risk factor for cardiac morbidity in the perioperative period [22, 23].
Hypertension has also been associated with cardiac morbidity in the perioperative period. An EKG that shows left ventricular hypertrophy with a strain pattern suggests a chronic ischemic state. Therefore, these patients are considered at a higher risk for perioperative cardiac morbidity. Hypertension should be aggressively treated as this has been shown to improve long-term risk [5, 24].
Assessing the need for further cardiac evaluation is based on the patient’s clinical risk predictive factors gathered from the preoperative evaluation and the risk associated with the particular surgery [25]. Possible intervention is based on the recommendation that only if the patient would benefit regardless of any planned non-cardiac surgery [23, 26].
Pulmonary Evaluation
Pulmonary dysfunction is the most common perioperative complication. A thorough assessment of the pulmonary status of the patient prior to surgery is essential as the impact on respiratory dysfunction is predictable. The failure to recognize patients who are at increased risk of perioperative pulmonary dysfunction may have significant morbidity and mortality. Failure to recognize can impact patient care intraoperatively; the patient may develop bronchospasm, atelectasis, and pneumonia and may require postoperative mechanical ventilation.
The pulmonary evaluation should encompass a detailed history of the patient’s pulmonary disease, type and severity, and possible reversibility. An account of the current medications used as well as steroid use, can be used to determine the proper perioperative regimen, and change in medication if necessary. The ASA has listed potential patient-related factors for the development of postoperative pulmonary complications. These include smoking, chronic obstructive pulmonary disease (COPD), obesity, ASA class >II, age >70, and prolonged general anesthesia >3 h. The patient should also be evaluated for obstructive sleep apnea and treatment instigated if necessary. This condition is associated with intermittent airway obstruction and increased difficult airway. Some patients have their own continuous positive airway pressure (CPAP) machine at home which they should bring for use in the postoperative setting. Patients should be evaluated for symptoms of cough, wheezing, dyspnea, productive sputum, as these patients may require further pulmonary testing. These may include chest radiograph, pulmonary function testing, arterial blood gas, and a preoperative pulmonary consult. There are no guidelines that define the degree of pulmonary function impairment that would prohibit surgery, except for lung resection. The pulmonary function tests that are associated with increased pulmonary complication in the perioperative period include; FEV1 <2 L, MVV <50 %, of predicted value, PEF <100 or 50 % of predicted value, pCO2 ≥45 mmHg, and pO2 ≤50 mmHg. Patients with known pulmonary disease should have their pulmonary function optimized before surgery. Careful consideration should be given to the choice of anesthesia, general vs. regional [5, 27].
Smoking is well known to increase risk in the perioperative period. The value for smoking cessation preoperatively has been evaluated. Cessation for 24 h prior to surgery has been shown to correlate with an improvement in hemoglobin, reduction in carbon monoxide, and improvement in oxygenation. Smoking cessation for more than 6 weeks has been shown to restore ciliary function back to baseline as well as improve oxygenation [9].
The patient with a recent upper respiratory infection poses a dilemma. If surgery is urgent or emergent then it should proceed. If surgery is elective the infection should be treated and surgery postponed. However the risk of delaying surgery should be weighed against the risk of proceeding with surgery at an increased risk of perioperative complications. The patient should be educated on the importance of smoking cessation and well as taught lung expansion maneuvers using incentive spirometry as risk reduction strategies in the preoperative period.
Other Organ Systems
The patient should be evaluated for organ dysfunction that can have an impact in the perioperative period. Liver disease may impact the clearance of anesthetic drugs. Coagulation factors are produced in the liver and hepatic dysfunction may impact coagulation in the perioperative period. Renal disease can severely impact the perioperative period as many anesthetic drugs are cleared via the kidneys. Chronic renal failure can impact clearance of anesthetic drugs as well as fluid management. Patients with chronic renal failure may need hemodialysis in the perioperative period. Complications from chronic renal failure include congestive heart failure, altered platelet function, hyperkalemia and other electrolyte disturbances, and chronic anemia. Patients must be evaluated for coagulation disorders as appropriate treatment may be instituted in the perioperative period and medications which may alter platelet function such as nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided. Those patients with thyroid disease may benefit from an adjustment in medication prior to surgery as hypothyroidism or hyperthyroidism may have a significant impact on perioperative morbidity. Surgery may need to be postponed until medication is adjusted, and thyroid function test is within normal limits [1, 28].
Nutritional assessment is an important part of the preoperative evaluation. Malnourished patients are at an increased risk for perioperative morbidity and mortality [29]. Risk factors for malnutrition should be evaluated especially in the elderly population. Risk factors such as chronic disease, poor dentition, limited financial resources, social isolation, depression, and chronic constipation–diarrhea are common in the elderly and are associated with poor nutrition status [30]. In addition anxiety related to surgery can lead to increased periods of non-eating and can further increase the malnourished state. An albumin level <3.2 g/dL is associated with malnutrition, and a lymphocyte count <3,000 μg/L is associated with increased postoperative complications [29–31].
Reducing the Risk of Perioperative Infection
Antibiotic prophylaxis in the perioperative period is an important modality in preventing surgical site infections [32]. This is of great importance to the anesthesiologist as urologic procedures involve deep surgical sites which if manipulated while infected can induce urosepsis within a few minutes and place the patient in a life-threatening situation [33]. The timing of antibiotic administration has been greatly debated over the years, and studies have demonstrated significant variability in the timing of prophylactic antibiotic administration. Postoperative infections are associated with high morbidity and mortality and add significantly to health care costs [34]. Specific antibiotic prophylaxis is required in patients with implantable devices such as prosthetic heart valves, coronary stents, automatic implantable cardiac defibrillator (AICD), and cosmetic implants. Prophylactic antibiotics administration is best given 1 h prior to surgical incision. This has shown to best correlate with reduction in the incidence of postoperative infections [34–36].
Patients taking anticoagulant therapy for chronic atrial fibrillation, prosthetic valves, coronary stents, and those who are at increased risk of developing thromboembolic events are at increased risk of bleeding during surgery. Warfarin, which decreases the synthesis of vitamin K dependent factors, should ideally be stopped 5–7 days before surgery. The day of surgery coagulation studies should be repeated and if normal surgery may proceed. Warfarin may be reversed with vitamin K administration, FFP, cryoprecipitate, or rFVII [37, 38]. Clopidogrel is an antiplatelet agent, which blocks amplification of platelet activation by released ADP. It should be stopped ideally 3–5 days before surgery. The effect of clopidogrel can be reversed with platelet transfusion, and there have been case reports that methyl prednisolone is effective as well [37, 39].
Fondaparinux is a synthetic pentasaccharide Factor Xa inhibitor and forms the high affinity binding site for the anticoagulant factor antithrombin III (ATIII). It does not inhibit thrombin, has a lower risk for heparin-induced thrombocytopenia (HIT) than low molecular weight heparin (LMWH), and is contraindicated in patients with renal dysfunction and in patients with body weight less than 50 kg [40]. There have been case reports of fondaparinux being used to anti-coagulate patients with established HIT. Fondaparinux has been shown to be similar to enoxaparin in reducing the risk of ischemic events at 9 days and to improve long-term morbidity and mortality [41]. The therapeutic effect of fondaparinux can be monitored with a factor Xa assay. There is no known reversal agent for fondaparinux. In the event of overdosage associated with bleeding complications, the drug should be discontinued and supportive care provided. The mean half-life of fondaparinux is 17–21 h. Fresh frozen plasma is ineffective in reversal and should not be used for this purpose [40, 42].
LMWH include Lovenox (Enoxaparin) and Fragmin (Dalteparin). They act by binding to, and enhancing the activity of, antithrombin III, thereby inhibiting the activity of activated clotting factors including thrombin (factor IIa) and activated factor X (factor Xa). Both medications preferentially inhibit Factor Xa while only slightly affecting clotting time. LMWH is dosed by weight and is contraindicated in patients with a body weight of less than 50 kg. Monitoring of LMWH is not necessary. However, if there is a concern for bleeding, or there is uncertainty about the therapeutic level, an LMWH blood assay is available. The therapeutic interval ranges between 0.5 and 1.1 U/mL. Hemorrhagic complications of therapy can generally be treated by stopping the medication. The mean half-lives for Lovenox and Fragmin are 4.5 and 2.2 h, respectively. Protamine sulfate can be used to reverse the effects of LMWH. The recommended dose is 1 mg protamine for every 1 mg Lovenox or 1 mg protamine for every 100 anti-Xa IU of Fragmin. Fresh frozen plasma is ineffective in reversal of LMWH to achieve hemostasis and should not be used for this purpose [43].
A new class of oral anticoagulants was approved by the FDA in 2010, as an alternative to warfarin in terms of dosing, monitoring, dietary concerns, drug interaction, and variability of response. Dabigatran (Pradaxa) and rivaroxaban (Xarelto) are direct thrombin inhibitors [44, 45]. These drugs target the tissue factor/Factor VIIa complex (Factors IXa or Xa, or their respective coenzymes, Factors VIIIa and Va), preventing the initiation of coagulation. They do not require frequent blood tests for international normalized ratio monitoring and offer similar results in terms of efficacy [46]. Dabigatran and rivaroxaban are contraindicated in patients with a creatinine clearance less than 30 mL/min. No specific modality exists to reverse the anticoagulant effect of both drugs in case of a major bleeding event [47, 48]. However, in the event of overdose within 2 h, activated charcoal may be given to reverse its effect. In the event of severe bleeding, dabigatran and rivaroxaban should be discontinued and supportive measure should be instituted: control of bleeding site, IVF, and blood monitoring with aPTT and TT. Monitoring with aPTT is used as a negative predictive value, where a normal value suggests little anticoagulant activity. TT monitoring is a sensitive tool and a normal value denotes no presence of anticoagulant [49, 50]. The administration of prothrombin complex concentrate (PCC) and rFVIIa should be used as a last resort. In renal failure patients, dabigatran’s half-life is prolonged to more than 24 h and may be eliminated up to 60 % with hemodialysis. Rivaroxaban is highly protein bound and is not eliminated with hemodialysis. Dabigatran and rivaroxaban should be discontinued 3–5 days before surgery. Patients requiring anticoagulation therapy may be admitted to the hospital and bridged with heparin infusion, which may be stopped 3–4 h prior to surgery [39, 44, 46, 51].
Preoperative Testing
The goal of preoperative testing is to detect asymptomatic disease processes not apparent on a history and physical examination. In the latter half of the twentieth century this was performed routinely in otherwise healthy individuals. The widespread use of laboratory testing was thought to detect those individuals who had latent disease processes and treat them before surgery in order to minimize morbidity and mortality in the perioperative period. Clinical effectiveness and cost effectiveness were not evaluated [52–54].
In the 1980s, several studies showed that routine preoperative testing was not cost-effective and did not influence perioperative management nor decrease perioperative morbidity and mortality [55]. Subsequent studies have shown that the majority of preoperative testing was done without any clear indications and that only a small percentage of patients had abnormal test results. This however, did not influence nor change their perioperative management. The problem lies in the ability of such tests to detect disease processes in otherwise healthy persons in whom the prevalence of such illnesses is low [56].
In patients who are more than 70 years of age, there is increased morbidity and mortality and increased hospital length of stay. However, in this age group there is also a greater incidence of comorbid conditions, and it remains unclear if the increased morbidity and mortality is secondary to age or to the comorbid conditions [7, 8].
The current recommendations for preoperative testing are based on specific indications in a patient. This greatly reduces the number of tests ordered. The following summarizes the recommended preoperative laboratory testing based on findings from the history and physical examination [52, 56, 57].
In healthy patients ≤40 years of age a baseline hemoglobin should be performed. In patients >40 year of age add an ECG and blood glucose (age ≥45 years) should be checked.
In patients with a history of cardiovascular disease, an ECG, chest radiograph, hemoglobin, serum electrolytes, BUN, and creatinine should be performed.
In patients with a recent MI (≤6 weeks), unstable angina, decompensated chronic heart failure (CHF), significant arrhythmias, severe valvular disease, and a cardiology consultation should be obtained [22]. If the patient suffered an MI more than 6 weeks ago or has a history of mild stable angina, compensated CHF, and/or diabetes mellitus, a stress test is indicated. If the patient has low functional capacity, consider echocardiography to assess left ventricular function [24, 58].
In patients with a history of pulmonary disease, the following should be obtained: chest radiographs hemoglobin, glucose (age ≥45 years), and ECG (age >40 years). Patients should be provided instructions for incentive spirometry or deep-breathing exercises. In asthmatics consider pulmonary function testing or peak flow rate to assess disease status. In patients with COPD consider pulmonary function testing and arterial blood gas analysis for assessment of disease severity. Any patient with a cough or dyspnea should be evaluated for etiology of symptoms. Patients who are current smokers should be counseled to stop smoking 4–8 weeks before surgery and provided with instructions for incentive spirometry or deep-breathing exercises. If malnutrition is suspected an albumin and lymphocyte count should be obtained; if malnutrition is severe, consider postponing surgery and providing preoperative supplementation [52–54, 57].
Intraoperative Management
Anesthetic Concerns
The anesthetic concerns regarding prostate surgery relate to positioning and ensuing hemodynamic changes. Whether the procedure is an open radical prostatectomy or performed as a minimally invasive surgery (MIS; laparoscopy with or without robotic assistance) ensuing physiologic changes that the patient encounters can significantly impact intraoperative management and perioperative morbidity [59, 60].
The decision as to the type of anesthesia given is first determined by the surgical procedure itself. Open RP may be performed under general anesthesia, regional anesthesia with sedation, or a combined general/regional technique. Regional anesthesia has been associated with less intraoperative blood loss, lower incidence of postoperative thromboembolic events, less incidence of postoperative nausea and vomiting (PONV), faster postoperative recovery, less postoperative mortality, and lower cancer recurrence rate [61]. Advocates of general anesthesia consider better airway control, greater intraoperative comfort, less intraoperative hemodynamic and pulmonary compromise, and less anesthesia time. When comparing general versus regional anesthesia, there is no difference in postoperative morbidity and no difference in outcome 3 months post-surgery [61–63].
Laparoscopic- and robotic-assisted RP (subsequently referred to as MIS) are preferably performed under general anesthesia, as under regional anesthesia, there is referred pain from the diaphragm that is not blocked using a regional technique. MIS surgery reduces blood loss and thus requires less intraoperative fluid replacement related to the increased risk in regards to positioning issues. In the post-anesthesia care unit (PACU), the immediate recovery period is noted for lower pain scores utilizing the ten point pain scale, less opioids administration, less PONV, and decrease PACU length of stay. The period after PACU discharge is significant for less postoperative complications, better hematocrit and platelet scores on discharge from the hospital, as well as decreased hospital length of stay. The laparoscopy approach has shown as well to be less stressful for the patient [3, 58–60].
The patient should have the standard monitors for general anesthesia. Additional intravenous access and placement of an arterial line should be a consideration depending on the patient’s comorbid condition. This is a concern especially in the elderly and the high-risk patient.
Open RP has the potential for rapid blood loss especially from the dorsal vein complex [64]. Our preference is to perform low-volume general anesthesia in which intravenous fluid administration is kept to a minimum until the prostate is removed in order to keep venous pressure low. Once the prostatectomy is completed fluids are given to replace blood and insensible fluid losses. Fluid restriction may also be helpful in MIS to reduce orbital, pharyngeal and laryngeal edema that may occur due to the Trendelenburg position. MIS has less blood loss than open RP with minimal insensible or third spaces losses. As such, MIS patients typically receive low volumes of fluid replacement. One concern, however, is decreased renal perfusion caused not only by restricted fluid replacement but also increased intra-abdominal pressure from the pneumoperitoneum and from activation of the sympathetic nervous system which causes an increased release of catecholamines and vasoconstriction of the afferent vessels to the glomeruli. This may increase creatinine levels measured in the postoperative period. In the absence of renal disease this is transient and has not shown to affect hospital length of stay and discharge [65]. On the other hand, the resultant oliguria may be interpreted as volume depletion and fluid may be given too generously. This can have a significant impact leading to pulmonary edema even in healthy patients. In the cardiac patient especially, this is a great concern as heart failure can develop acutely. Fluid replacement should be given cautiously is this patient population.
Additional anesthetic concerns with MIS are related to the effects of pneumoperitoneum and steep Trendelenburg position that affects homeostasis of most organ systems of the body.
Cerebral Effects
The cerebral perfusion pressure (CPP) is the driving pressure for brain perfusion and can be expressed as CCP = MAP (mean arterial pressure)—(CVP) central venous pressure. The head down position in steep Trendelenburg increases arterial and central venous pressure (CVP), thus impending venous flow from the brain and increasing hydrostatic pressure within the brain vasculature [66]. There is an increase in perivascular edema and the extracellular water content of the dependent tissues which may lead to cerebral edema. The resultant increase in intracranial pressure (ICP) can increase cerebrovascular resistance and reduce blood flow. In patients with cerebrovascular disorders these changes can lead to impaired tissue oxygenation, impaired perfusion and diffusion, and disastrous consequences. The brain is also very sensitive to CO2. Hypercarbia causes direct vasodilation which also increases ICP. Slight elevations can cause direct cortical depression and decrease the threshold for seizure activity. This hypersensitivity is enhanced by hypercarbia-induced stimulation of the hypothalamus from catecholamines released from the medulla of the adrenal gland. Increased intra-abdominal pressure causes increased intrathoracic pressure which causes a direct increase in ICP by the mechanisms described above. The added hypercarbia contributes as well to increase ICP, but hyperventilation will not cause a decrease in intracranial pressure until the abdominal pressure is released. Although all these various factors described contribute to an increase in intracranial pressure, this is well tolerated by the ASA class I or ASA class II patient. The use of a cerebral oxygen monitor during steep Trendelenburg position has shown that there is a slight increase in regional cerebral oxygen saturation with increased CO2 in healthy patients. Consequently, the PaCO2 should be maintained within normal ranges during the steep Trendelenburg position [66, 67].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree