Angiographic, Venographic, and Hemodynamic Evaluation of Portal Hypertension
Portal hypertension can be defined as the persistent elevation of portal pressure in excess of 25 to 30 cm of saline with decreased flow in the major portal branches, development of numerous portosystemic venous collaterals, and passive congestion of the spleen and other viscera.1 The most common presentation of portal hypertension is painless massive hematemesis or melena without obvious precipitating cause. When cirrhosis progresses to the stage at which portal hypertension becomes symptomatic, angiography is frequently used in the evaluation of these patients to document alterations in blood flow and to quantify, both subjectively and objectively, changes in the normal hemodynamics of the portal venous system. Because hemodynamic changes are intimately related to the severity of the disease, angiography, although invasive, is a primary means of acquiring necessary physiologic data for planning both nonsurgical and surgical interventions. In addition, angiographic evaluation can (1) aid in the differentiation between the types of portal hypertension, (2) aid in determination of the etiology, (3) assess the portal venous system for patency before intervention, (4) evaluate the liver for incidental hepatocellular carcinoma (3.5–30%),2,3 and (5) determine the feasibility of variceal embolization before initiation of primary therapy or as the treatment of choice.4–9
A number of angiographic techniques are available for evaluation of the portal venous system and associated hemodynamic alterations common to cirrhosis and portal hypertension.
Each of these procedures has a different role in the evaluation of patients with portal hypertension. This chapter covers these techniques and the methodology paramount to optimize portal venous system evaluation.
 Patient Preparation
Patient Preparation
Arterial portography and hepatic venography with hemodynamic assessment typically are performed in the stable patient with portal hypertension in whom evaluation of the portal venous system is required. Standard preparation of the patient, as for any patient undergoing arteriography, is necessary with two particular thoughts in mind. Patients with advanced cirrhosis typically have decreased hepatocyte function, and clearance of specific drugs may occur at a decreased rate. Thus, caution must be exercised not to overmedicate the cirrhotic patient with preprocedural and intraprocedural drugs that require hepatic detoxification (i.e., fentanyl citrate, morphine sulfate). Also, abnormal coagulation parameters may be seen in patients with moderate to advanced disease. Hypoprothrombinemia can occur secondary to decreased synthetic function, and platelet counts can be abnormally decreased secondary to sequestration from hypersplenism.1 Knowledge of the prothrombin time (PT), activated partial thromboplastin time (aPTT), and platelet count are thus imperative. Correction of the PT and aPTT to within 1.3 times control in addition to a platelet count greater than 50,000/μL is advised before an invasive evaluation is initiated.
 Arterial Portography
Arterial Portography
Technique
Arteriographic evaluation usually is performed from a transfemoral approach, but in patients with limited access sites or in patients with preplaced sheaths available for use, a transjugular or brachial approach also can be used. Using the Seldinger technique, a 5F angiographic catheter (Cobra or sidewinder configuration) is advanced into the abdominal aorta, and the celiac axis is selected. An injection rate of 7 mL per second for a total of 50 mL of contrast (diluted to about 75% with normal saline) is usually adequate in most patients for digital subtraction angiography (DSA) of the splenoportal axis. Patients with splenomegaly may require a larger volume of contrast (up to 70 mL) or a direct splenic artery injection to obtain adequate opacification of the splenic and portal veins. A rate increase of 0.3 second will decrease catheter recoil without affecting film quality. Filming is at one image per second.
Following completion of the celiac injection, the catheter is moved to the superior mesenteric artery (SMA) for the second part of the examination. An injection rate of 7 mL per second for a total of 70 to 80 mL of contrast is used following the direct injection of 25 to 50 mg of tolazoline (diluted in 10 mL normal saline) into the SMA through the catheter. The dose of tolazoline should be titrated or eliminated in patients with hypotension. Imaging parameters are the same as those used for the celiac axis.
Arterial Phase
The arterial phase of each examination should be evaluated first. The splenic artery will most likely be normal in appearance in cases with minimal disease; however, it may be dilated and extremely tortuous, depending on the inflow and degree of outflow obstruction. Aneurysms of the splenic vessels distal to the hilum may be demonstrated. The normal liver receives abut 20% of its blood supply from the hepatic artery and 80% from the portal vein. The size of the hepatic artery can be variable. In cases in which the portal venous resistance is low to normal, the hepatic artery typically has a normal appearance, or about one-third the diameter of the adjacent portal vein. As the portal venous resistance increases, the hepatic perfusion is compensated for by increased hepatic artery inflow. Dilatation of the hepatic artery can thus occur, and increased opacification of the liver during the parenchymal phase of the examination may be seen. In cirrhotic patients, the hepatic artery diameter may increase to two-thirds of the diameter of the adjacent portal vein. The size and number of intrahepatic arteries visualized also will increase.10 As cirrhosis progresses, “corkscrewing” of the intrahepatic branches occurs as the liver shrinks.
Regenerating hepatic nodules and hepatocellular carcinoma can occur with some frequency in the cirrhotic liver. The regenerating nodule is distinguished from a hepatocellular carcinoma by its lack of distorted, tortuous, and irregular vessels and by the absence of contrast puddling and arterioportal shunting. Rather, normal-appearing vessels contributing to an area of hypervascularity are characteristic of the regenerating nodule.11
Parenchymal Phase
An inhomogeneous parenchymal phase can be indicative of fatty replacement and does not necessarily indicate advanced cirrhosis. Selective left gastric artery injections are usually not required for a complete evaluation but may provide for better opacification and determination of flow direction within gastroesophageal varices, particularly in cases with inconclusive findings on arterial portography. The arterial phase of the SMA injection is usually unremarkable.
Portal Venous Phase
The portal venous phase following each arterial injection typically demonstrates the hallmark features key to the angiographic diagnosis of portal hypertension. The portal venous phase is accentuated during the SMA injection with the use of tolazoline, which often results in dramatic demonstration of the mesoportal axis, varices, and alterations in the normal blood flow patterns. The level of obstruction can be categorized as presinusoidal (extrahepatic and intrahepatic), intrahepatic (sinusoidal), and posthepatic (postsinusoidal).12,13 This section discusses in detail the hallmark features seen during angiographic evaluation of the various types of portal hypertension, in addition to alternate methods available for contrast evaluation of the portal venous system.
 Presinusoidal Extrahepatic Portal Hypertension
Presinusoidal Extrahepatic Portal Hypertension
Presinusoidal extrahepatic portal hypertension is most commonly due to portal vein obstruction, which, in the United States, is most frequently attributed to portal vein thrombosis (Fig. 2–1). This condition can be due to a number of common causes, including tumor, adenopathy, and benign masses such as a pancreatic pseudocyst or choledochal cyst. In cases of partial portal vein obstruction, some component of hepatopetal blood flow is usually preserved, and the portal vein will be (partially) opacified during the portal venous phase of the superior mesenteric artery injection (Fig. 2–2). Digital subtraction imaging typically allows visualization of the portal vein, even if blood flow is significantly decreased. Tolazoline enhancement will not uncommonly be required to optimize imaging. Hepatic artery inflow compensates for the decrease in portal vein supply to the liver. Splenic vein pressure is increased accordingly, depending on the degree of portal vein obstruction, and this is reflected in the splenic pulp pressure. Decompressive varices can develop and, depending on the degree and position of the obstruction, dilatation of the portal vein proximal to the obstruction may occur.
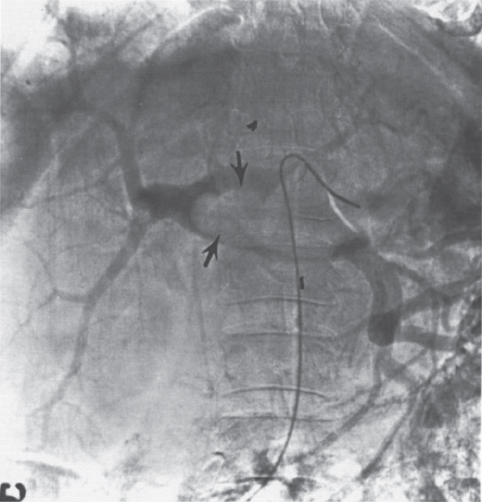
FIGURE 2–1. Portal venous phase of the superior mesenteric artery arteriogram in a patient with polycythemia vera rubra. Note the large filling defect (arrows) secondary to thrombus in the dilated portal vein.
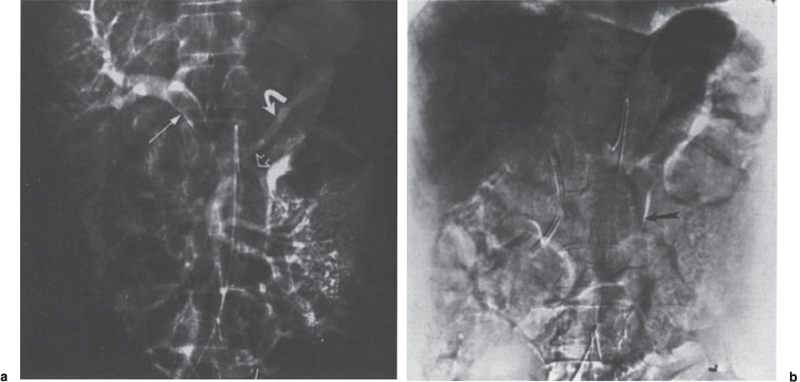
FIGURE 2–2. (a) Mucinous adenocarcinoma with portal vein invasion. Extensive tumor within the portal vein (arrow) is appreciated. In addition, there is hepatofugal flow in the inferior mesenteric vein (open arrow) and retrograde filling of the splenic vein (curved arrow). Note that despite the relatively large intraportal vein mass, there is excellent filling of the intrahepatic portal vein branches, (b) The late portal venous phase clearly identifies the hepatofugal flow in the inferior mesenteric vein (arrow). Note the relative homogeneity of the liver during the parenchymal phase resulting from good portal vein flow despite the presence of the obstructing lesion.
In total occlusion of the portal vein, hepatic artery inflow must compensate significantly for the decreased portal venous return. Portal blood flow to some degree, however, is maintained by hepatopetal collaterals, including the cystic, gastroepiploic, omental, hepatocolic, diaphragmatic, and periumbilical veins.11 The more common collateral pathways that may be visualized include the following:14
• Splenic vein →→ omental vein (arc of Barkow) portal vein
• Splenic vein →→ short gastric veins →→ left coronary vein →→ portal vein
• Splenic vein →→ gastroepiploic vein →→ portal vein
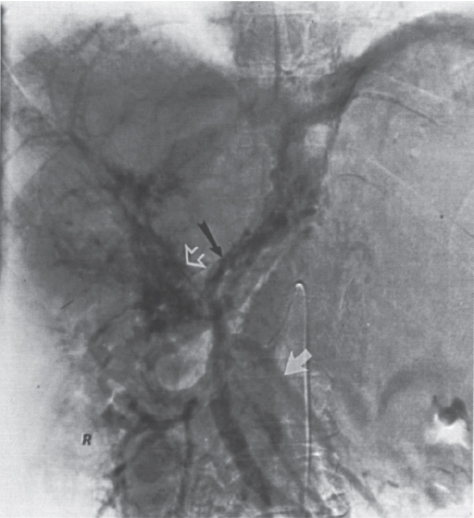
FIGURE 2–3. Cavernous transformation of the portal vein (open arrow) with splenic vein thrombosis in patient with polycythemia rubra vera. Note the hepatofugal flow in the left coronary vein (black arrow) and inferior mesenteric vein (white arrow).
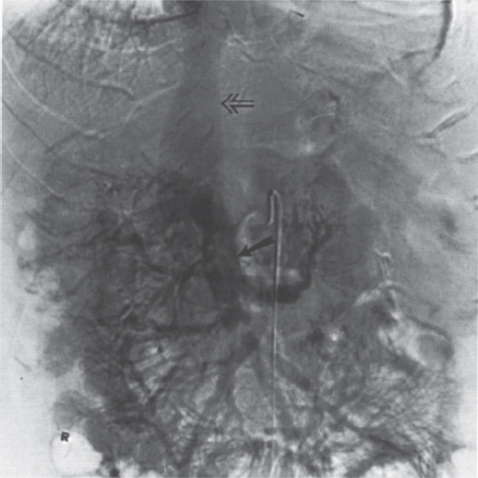
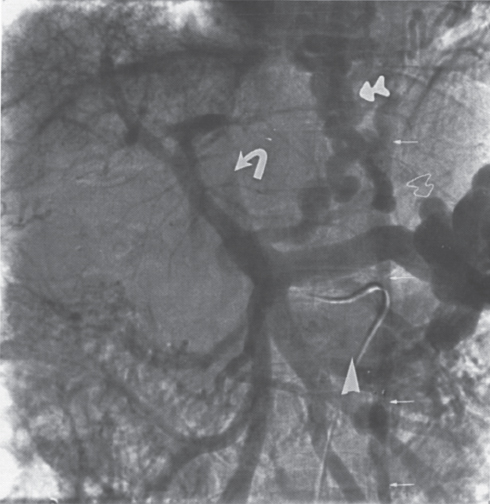
FIGURE 2–4. Portal hypertension secondary to hepatitis Cinduced cirrhosis. Note the shunt (veins of Retzius) occurring directly from the superior mesenteric vein (closed arrow) to the inferior vena cava (open arrow).
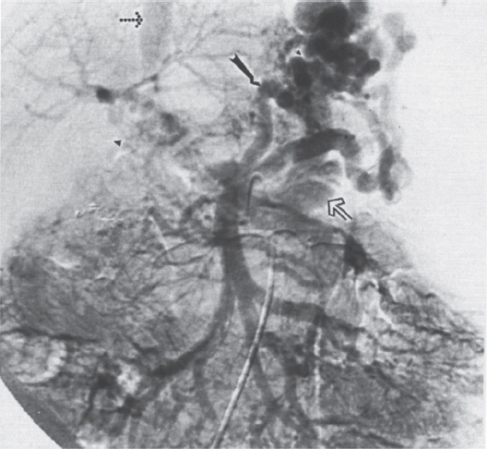
FIGURE 2–5. Spontaneous splenorenal shunt in patient with hepatic cirrhosis secondary to biliary atresia. The portal venous phase of the superior mesenteric artery arteriogram demonstrates hepatofugal flow in the left coronary vein (arrow) with filling of the left renal vein (open arrow) and inferior vena cava (dotted arrow). Note the cavernous transformation (arrowhead) of the thrombosed portal vein.
Cavernous transformation of the portal vein is due to the development of an extensive network of periportal veins (choledochal veins) within the portahepatis (Fig. 2–3), which may provide an additional route for significant blood flow to bypass an obstructed main portal vein. A less common means of portal vein decompression involves the development of middle and right colic to portal vein collaterals or direct superior mesenteric vein to inferior vena cava shunts (veins of Retzius) (Fig. 2–4).
Two recent studies on splenoportography documented a significant number of spontaneous splenorenal (27%) and splenoadrenal (9.4%) shunts on distal splenoportography and splenoportovenography, respectively, in patients with extrahepatic portal vein obstruction.15,16 In cases of spontaneous splenorenal shunts (Fig. 2–5), the incidence of variceal hemorrhage was 45.5% versus 54.5% in those without a splenorenal shunt.15,16 This shunt would appear to afford no significant protection against variceal hemorrhage.
 Other Forms of Presinusoidal Extrahepatic Portal Hypertension
Other Forms of Presinusoidal Extrahepatic Portal Hypertension
Artenoportal Shunting
Arterioportal shunting, or dynamic portal hypertension, results from an abnormal communication of arterial vessels with the intrahepatic or extrahepatic portal venous system. The communication may be congenital or acquired in nature.17
Patients with Osler-Weber-Rendu syndrome (hereditary hemorrhagic telangiectasia) have a 15% incidence of telangiectatic lesions in the intestinal tract. Vascular lesions, including telangiectasias, cavernous hemangiomas, hepatic artery aneurysms, and both hepatoportal and hepatic arteriovenous fistulas, also can occur within the liver.18 Various levels of shunting to the portal venous system can thus occur. The cumulative effect of these lesions, superimposed on hepatic fibrosis, can potentiate and exacerbate portal hypertension in this group of patients.19
Acquired aterioportal shunting can be the result of penetrating and blunt abdominal trauma.20–23 Stab wounds and bullet injuries to the abdomen or liver can result in arterioportal shunting that can progressively increase if not detected at the time of the initial insult (Fig. 2–6). Iatrogenic injuries occurring during surgery, liver biopsy, percutaneous biliary drainage, and percutaneous transhepatic left coronary vein embolization infrequently result in symptomatic arterioportal shunting.24–26 Studies in which hepatic arteriography was performed following needle biopsy, percutaneous transhepatic cholangiography, and percutaneous biliary drainage demonstrated a 5.4%, 1 to 3.8%, and 7 to 26% incidence of arterioportal fistula, respectively.26–29 Fortunately, few patients become symptomatic, and some of these lesions may even be self-resolving, particularly small peripheral lesions.
Neoplastic disorders are the most common cause of intrahepatic arterioportal shunts in the absence of any history of trauma. In a study by Adler et al,24 previous reports in the literature on intrahepatic arterioportal shunts were reviewed. Tumor was cited cumulatively as the etiology of the shunt in 46 (51%) of 90 cases. Hepatic artery to portal vein shunts may occur in up to 63% of hepatomas, and hemorrhage from esophageal and gastric varices will contribute to mortality in 25% of these patients.30 Hepatic artery ligation has limited treatment success because of the rapid formation of intrahepatic collaterals. Surgical portosystemic shunting not only fails to decrease the portal pressure but also can lead to high output heart failure.31,32 Morse et al3 demonstrated that percutaneous subselective hepatic artery embolization is effective in reducing or eliminating the arterioportal shunt, decreasing portal venous pressure, and terminating life-threatening hemorrhage from gastroesophageal varices.31 In a study by Tarazov,34 13 patients with hepatocellular carcinoma and arterioportal shunting were either treated conservatively (n = 8) or with transarterial hepatic artery embolization (n = 5). Of the patients treated conservatively, all died from massive variceal hemorrhage within 3 to 60 days of their diagnostic arteriogram. However, the patients treated with transarterial hepatic artery embolization survived a mean of 9.4 months, with a range of 2 to 30 months.33
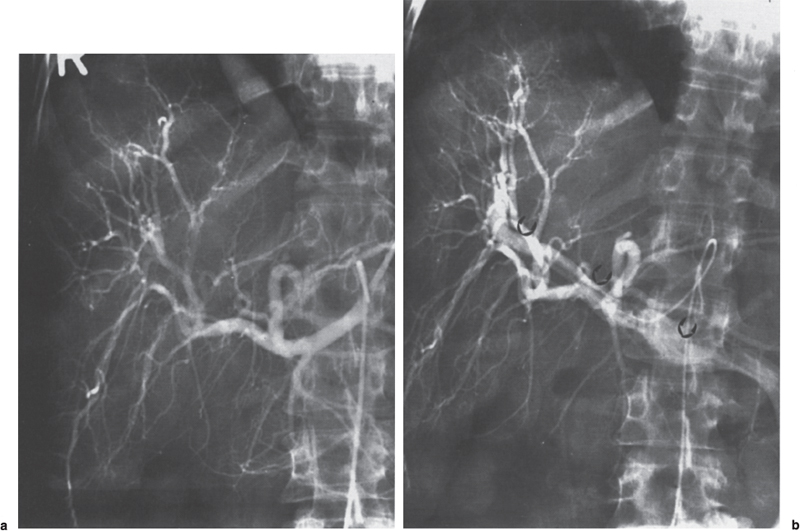
FIGURE 2–6. Intrahepatic arlerioportal fistula following stab wound, (a) The early arterial phase is normal, (b) The late arterial phase shows arterial branches and hepatofugal flow in the portal vein (arrows).
Regardless of etiology, if the vascular “injury” is not detected early, increasing arterioportal shunting can result in gradual elevation of portal-venous pressure with subsequent development of decompressive portal venous shunts. Selective and superselective catheterization with percutaneous embolization of arterial feeders should be the first line of defense. As demonstrated by Morse et al31 and Tarazov,34 percutaneous transarterial embolization has a high technical and clinical success rate in arterioportal shunts of neoplastic origin. This favorable outcome has been repeated with percutaneous embolization of fistulas of numerous other etiologies.22,26,30,33,35
Uncommon causes of arterioportal fistulae include inflammatory injury to arterioportal vessels (i.e., pancreatitis), hepatic artery aneurysm rupture into the portal venous system,36 congenital or iatrogenic splenic arteriovenous malformation, or fistulae, respectively,37 regenerating liver nodules and cirrhosis,38 metastatic disease,39 and schistosomiasis of the liver.40
Splenic Vein Thrombosis
Isolated splenic vein thrombosis (SVT) is an uncommon entity, with about 200 cases published in the English literature as of 1985.41,42 SVT results in what has been termed isolated or left-sided portal hypertension. Sutton et al41 reported pancreatic neoplasm to be responsible for 18.5% cases of SVT. Moosa and Gadd42 reported pancreatitis to be the etiology in 81 (56%) of 144 cases, with neoplasm a relatively uncommon cause of SVT. Other reported causes of SVT include trauma,43 umbilical vein catheterization,44 pancreatic pseudocyst or abscess,45,46 lymphoma,47 thrombocytopenia,48 iatrogenic injury during surgery,49 renal cell carcinoma and renal cyst,43,50 and pregnancy.51
Patient presentation is variable, and the diagnosis of SVT may be made in a large percentage of patients during evaluation for another disease (e.g., pancreatitis, pancreatic carcinoma).42,52 Abdominal pain may occur in up to 89%54 and iron-deficient anemia in 22%.53 Gastro-esophageal hemorrhage from varices occurs in 45 to 64% of cases.51,42,53 In a review by Moosa and Gadd,42 the distribution of varices was as follows: isolated gastric, 49%; gastroesophageal, 43%; and isolated esophageal, 8%. Thus, although the esophagus is often thought to be protected from variceal formation secondary to unobstructed hepatopetal flow in the left coronary vein, esophageal varices can occur as a component of the disease in almost 50% of patients. To complicate matters further, isolated gastric varices are more difficult than esophageal varices to identify on endoscopy because enlarged gastric folds may be interpreted as simply prominent or secondary to inflammation or infiltration (i.e., lymphoma). In one study, endoscopy was incorrect in identifying isolated gastric varices in 40% of cases42 and in a second study, correctly identified them in only 40% of cases.53 Angiography remains the “gold standard” for evaluation of SVT: It is diagnostic in essentially 100% of cases.42
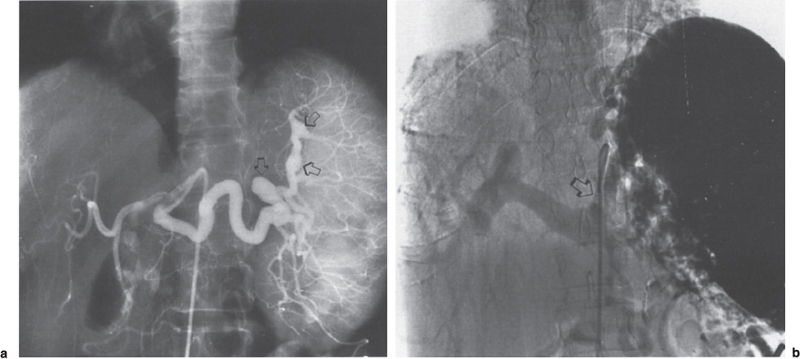
FIGURE 2–7. Splenic vein thrombosis in patient with portal hypertension and history of pancreatitis. (a) The celiac axis arteriogram demonstrates splenomegaly and aneurysmal dilatation of the splenic artery (arrows). (b) The portal venous phase demonstrates hepatopetal flow in the left coronary vein (arrow) without opacification of the splenic vein.
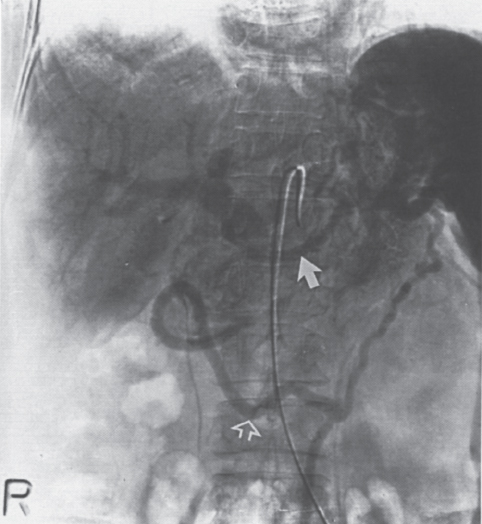
FIGURE 2–8. Splenic vein thrombosis secondary to pancreatic carcinoma. The thrombosed splenic vein is bypassed with flow through the gastroepiploic vein (closed arrow) and the transverse omental vein (arc of Barkow) (open arrow).
Arteriographic findings in SVT may include an enlarged and tortuous splenic artery in up to 50% of the patients, reflecting markedly increased flow.54
Nonvisualization of the main splenic vein, despite filling of splenic hilar veins, is the hallmark finding on the portal venous phase of the celiac or splenic artery arteriogram (Fig. 2–7). Splenomegaly is noted in only 50% of patients. Opacification of decompressive shunts originating from the splenic vein remnant or splenic hilum can include the following venous circuits:
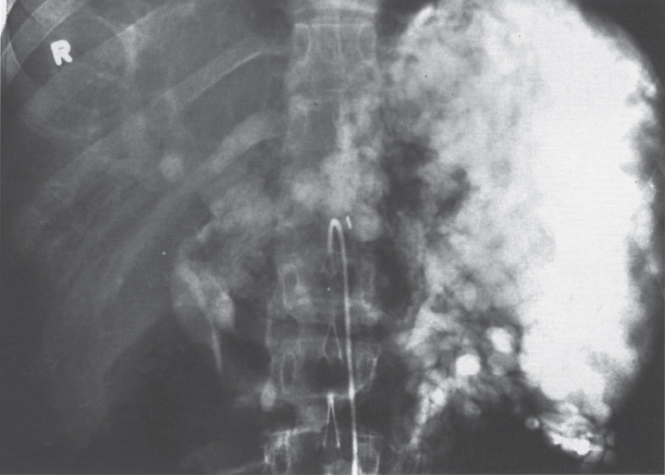
FIGURE 2–9. Splenic vein thrombosis of unknown etiology. This spleen is enlarged, and there are extensive varices throughout the region of the splenic hilum and in the expected region of the left coronary vein. There also appears to be cavernous transformation of the portal vein.
• Short gastric veins →→ esophageal veins →→ azygos system superior vena cava
• Short gastric veins →→ left gastric vein →→ portal vein
• Left gastroepiploic vein →→ right gastroepiploic vein →→ gastroduodenal veins →→ portal vein (Fig. 2–8)
• Transverse omental vein →→ gastroduodenal veins →→ portal vein (arc of Riolan) (Fig. 2–8)
• Inferior mesenteric vein →→ hemorrhoidal veins →→ pudenal veins →→ internal iliac veins
• Retroperitoneal veins including inferior phrenic, renal, and adrenal veins →→ inferior vena cava
The portal vein is typically patent, and flow is hepatopetal in direction. The wedged hepatic vein pressure is normal.
Splenic vein thrombis has been referred to as the curable variceal hemorrhage. Splenectomy has long been recognized to result in a permanent cure for the associated gastroesophageal bleeding.41,42,52,53,55,56,57 Because splenectomy is contraindicated in patients with concomitant portal vein thrombosis, however, an SMA injection in addition to the celiac axis study with attention to the portal vein is indicated in all patients undergoing angiographic evaluation (Fig. 2–9).
Superior Mesenteric Vein Thrombosis
Superior mesenteric vein thrombosis (SMVT) accounts for 15 to 55% of all mesenteric vascular occlusions.59 Since its first description in 1935 by Donaldson and Stout,60 numerous etiologies have been reported, including oral contraceptive use61,62; neoplastic disease, sepsis, cirrhosis, abdominal surgery,63 variceal sclerotherapy64; appendicitis65; trauma;66,67 polycythemia vera rubra68; and antithrombin III deficiency.69 Interestingly, a number of idiopathic cases have been described in association with deep venous thrombosis of the lower extremities.70
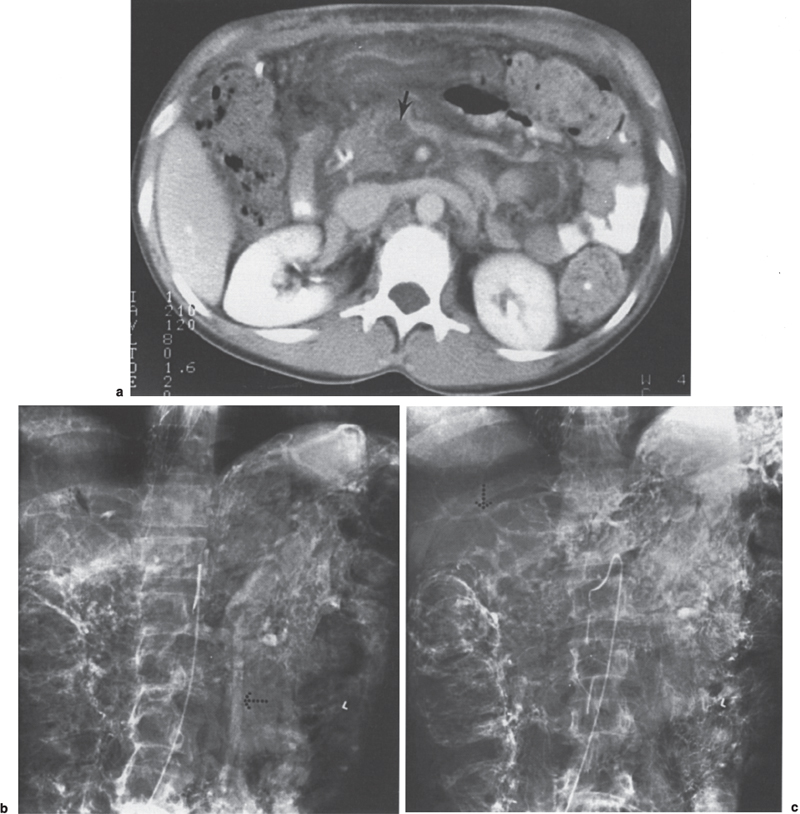
FIGURE 2–10. Superior mesenteric vein and extrahepatic portal vein thrombosis in patient with polycythemia vera rubra. (a) The computed tomography scan shows a low density mass (arrow) in the lumen of the superior mesenteric vein that is consistent with thrombus. Note the edematous appearance of the mesentery. (b) On the midportal venous phase, there is no opacification of the superior mesenteric vein; however, there is hepatofugal flow in the inferior mesenteric vein (dotted arrow). (c) In the late portal venous phase, there is opacification of the intrahepatic portal vein branches (dotted arrow).
Patients may present with abdominal distention and pain, severe guarding, anorexia, nausea, vomiting, diarrhea, hematemesis, melena, or shock.71–73 Mortality rates are, in part, dependent on the degree of SMVT and associated splanchnic vein thrombosis and bowel infarction. In a review, Gertsch et al72 analyzed the results of 64 reported cases of mesenteric venous thrombosis. Complete thrombosis of the superior mesenteric vein resulted in a mortality rate of 54%. Patients with SMVT and associated portal vein and thrombosus SVT had a mortality rate of 76%. Partial SMVT carried an 11% mortality rate.
The hallmark angiographic findings in SMVT are nonvisualization of the superior mesenteric vein on the venous phase of the superior mesenteric artery arteriogram. Opacification of numerous dilated jejunal and ileal veins and dilated omental collaterals are present, which drain into the splenoportal axis. The portal vein may be poorly opacified as a result of dilution of the radiographic contrast (Figs. 2–10).
The mortality rate without surgery initially was estimated to be close to 100%.74 More recent publications have shown that, in selected patients, anticoagulation,73,75 intravenous thrombolytic therapy,76 and direct transhepatic thrombolytic therapy77 all can result in a favorable outcome.
 Presinusoidal Intrahepatic Portal Hypertension (Idiopathic Portal Hypertension)
Presinusoidal Intrahepatic Portal Hypertension (Idiopathic Portal Hypertension)
Banti’s syndrome is a generic term for a group of diseases that all present with the similar findings of splenomegaly and hypersplenism, anemia, and portal hypertension.78 A proposed mechanism for these findings postulates that an immunologic response to an antigenic stimulus (i.e., Escherichia coli, vinyl chloride, or copper vapors) results in splenic sinus hyperplasia and splenomegaly. This entity has been described worldwide, but it is most common in India and Japan, where it has been termed noncirrhotic and idiopathic portal hypertension, respectively.79 Hepatoportal sclerosis is the term applied in the United States to the equivalent of this process.80 Patient presentation is typical of that associated with portal hypertension, that is, bleeding gastroesophageal varices; however, liver biopsies in these patients demonstrate histologic findings atypical of those seen with alcoholic cirrhosis. In addition, when these patients are followed up for an extended period, the clinical course is also different than that typical of alcoholic cirrhosis. In patients with idiopathic portal hypertension in whom bleeding is controlled relatively well, the 10-year survival rate is 77% versus 29% for cirrhosis in general.80
The livers in patients from India and Japan with idiopathic portal hypertension have been compared, and the findings have been shown to be quite similar,81 thus suggesting a common mechanism of injury though the inciting event may be different. On a microscopic level, pathological changes include asymmetric hepatic atrophy, fibrosis of the portal tracts, phlebosclerosis of the portal vein wall and branches with thrombosis, a paucity of middle-sized intrahepatic portal vein branches, microscopic portal scarring, and obliteration of small portal radicles.81 Because the portal vein radicles (venules) are the outflow tract that connect the portal vein to the sinusoids and central vein, fibrosis of the portal vein venule results in impaired outflow of blood through normal anatomic pathways. Because the sinusoids and central vein are not impaired, the reduced portal vein inflow is compensated for by increased hepatic arterial inflow. Eventual involvement of the hepatic lobule, sinusoids, and central vein does occur late in the disease, which can render the histologic picture similar to that seen in advanced alcoholic cirrhosis.82–85
The angiographic findings of dramatic splenomegaly, a patent splenic vein, and decompressive variceal shunts are the radiographic hallmarks of idiopathic portal hypertension on radiographic evaluation (Fig. 2–11). Collaterals develop in a pattern similar that seen with cirrhosis, and no significant differences in distribution are noted, although recanalization of the umbilical vein is rarer.86 The portograms, however, are distinctly different and show the following findings: (1) a paucity of middlesized portal vein branches (78%), (2) an absence or abrupt termination of peripheral portal vein branches (65%), (3) a region of hypervascularity in the immediate subcapsular regions (43%), and (4) a peripheral contrast “blush” without definition of small-caliber portal vein radicles (27%).86 Portal vein thrombosis has been noted in up to 13% of patients.80
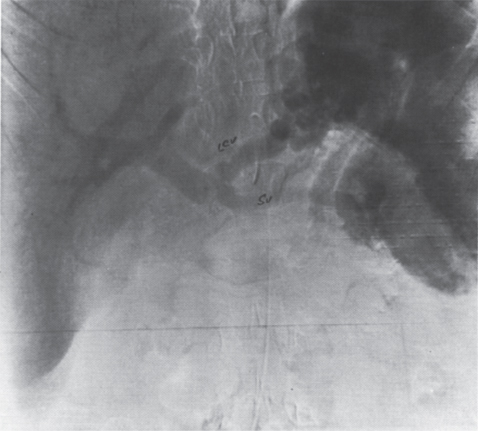
FIGURE 2–11. Young female patient with multiple postpartum gastroesophageal hemorrhages. The portal venous phase of the celiac arteriogram demonstrates a patent splenic vein (SV) and a patent left coronary vein (LCV). Splenomegaly is also present.
 Intrahepatic Sinusoidal Portal Hypertension
Intrahepatic Sinusoidal Portal Hypertension
Alcoholic cirrhosis is the most common cause of intrahepatic portal hypertension occurring at the level of sinusoids. Alcoholism affects 10 million people in the United States and leads to 200,000 deaths per year, making alcoholic liver disease the fourth leading cause of death among persons aged 25 to 64 years in the United States.88 On a histologic level, the portal zones, sinusoids, and hepatic venules are involved. Cellular injury, predominantly in Rappaport zones 2 and 3, and subsequent repair with hyalin deposition and fibrosis result in obliteration of venous outflow from the hepatic lobule.14,88 Early in the disease (stage I), arterial portography may demonstrate normal anatomy and flow. In moderate disease (stage 2), some portosystemic collaterals develop to compensate for the increased intrahepatic resistance. The left coronary vein is a common decompressive pathway: Blood flows in a hepatofugal direction to the gastroesophageal venous plexus (Fig. 2–12). The hepatic artery responds to the decreased portal venous supply to the liver with a compensatory increase in blood flow. Enlargement and tortuosity of the hepatic artery may occur. The pancreaticoduodenal arcade also may be recruited to supply blood to the liver. Splenic artery aneurysms can also occur in patients with alcoholic cirrhosis (Figs. 2–7a and 2–13a,b).
As the portal venous resistance continues to rise, portal venous blood flow may become stagnant or reversed (stage 3), and numerous additional portosystemic shunts develop that commonly include the hemorrhoidal, mesenteric, retroperitoneal, umbilical, and periumbilical veins (Figs. 2–14). Intrahepatic portosystemic shunting from the portal vein directly to the hepatic vein or inferior vena cava also has been reported (Fig. 2–15).89,90 This particular group of patients commonly (69%) present with neurologic findings compatible with hepatic encephalopathy. Symptoms include disorientation, abnormal behavior, and loss of consciousness. Laboratory studies show grossly elevated serum ammonia levels.91
Clinically, the left coronary vein is the most significant decompressive shunt because of the high incidence of gastroesophageal hemorrhage. Because of the potential for therapeutic embolization, accurate determination of collateral pathways from the left coronary vein is important. Left coronary vein varices may be present in up to 95% of patients with portal hypertension, and multiple left coronary veins may occur in up to 24% of patients.92 In a study by Widrich et al,92 spontaneous communication of the left coronary vein with systemic veins was identified in 46% of patients who underwent selective left coronary vein venography. Routes of these decompressive shunts include the following:
• Left coronary vein →→ left inferior phrenic vein →→ left renal vein, 66%
• Left coronary vein →→ pericardiophrenic vein, 10%
• Left coronary vein →→ left portal vein, 7%
• Left coronary vein esophageal varices →→ pulmonary vein, 6%
• Left coronary vein right inferior phrenic vein, 4%
• Left coronary vein →→ inferior vena cava, 3%