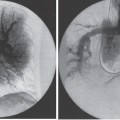
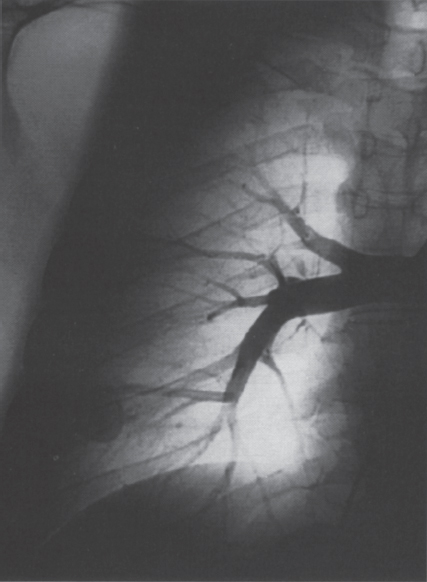
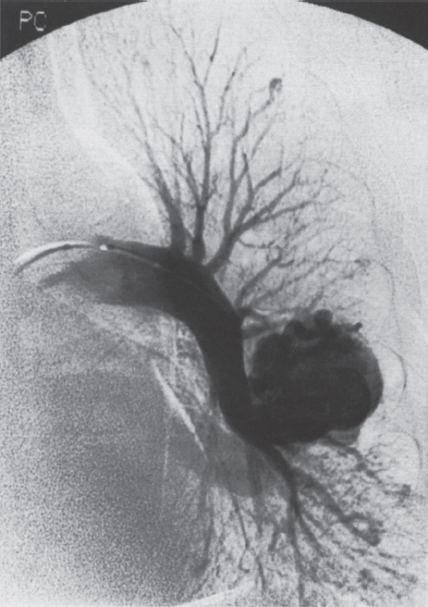
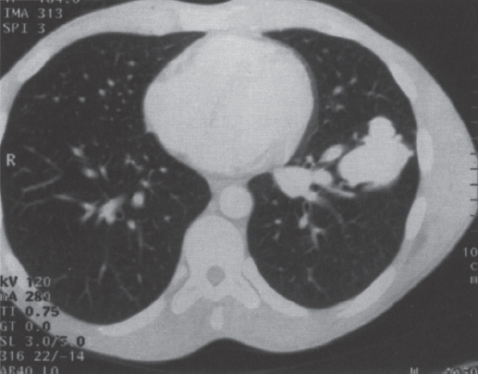
Pulmonary Arteriovenous Malformations Pulmonary arteriovenous malformation (PAVM) is a rare disorder that involves a direct low-pressure abnormal communication between the pulmonary arterial and venous circulation. The often complex nature of the lesion has given rise to various descriptive pathologic terms, such as pulmonary arteriovenous fistula, pulmonary arteriovenous aneurysms, pulmonary angiomas, and telangiectasia.1–6 These congenital malformations originating from abnormal development of the pulmonary capillaries have a high degree of association with Osler-Weber-Rendu, also known as hereditary hemorrhagic telangiectasia (HHT). Hereditary hemorrhagic telangiectasia is an uncommon autosomal dominant multiorgan disorder that directly affects the lungs, nose, and gastrointestinal tract. Patients diagnosed with this rare disorder can carry a mortality risk of 10% related to the serious systemic manifestations of the disease process.7 Patients who are asymptomatic at the time of diagnosis often have undergone investigation for an unexpected lesion on a chest radiograph. Symptomatic patients may have profound dyspnea, cyanosis, and clubbing. In patients with documented PAVM, treatment is directed to closure of the shunt, thereby significantly reducing morbid features of the disorder, such as stroke or brain abscess, occurring as a result of paradoxical embolization. Neurologic complications not only give rise to significant morbidity but also are the leading cause of death in patients with PAVM and HHT. Although surgical intervention with thoracotomy and conservative lung-sparing resection has been effective in altering the natural history of the disorder, percutaneous transcatheter embolization techniques pioneered by Taylor et al8 and Terry et al9 have provided other safe and effective modes of therapy. Increasing experience with embolotherapy over the last 20 years and several long-term follow-up studies by Remy et al10 and Lee et al11 confirmed the durability and efficacy of this procedure. Because many patients may have bilateral pulmonary malformations and may require multiple procedures over their lifetimes because of the growth of smaller malformations, transcatheter therapy should be the definitive treatment for PAVM. With readily available selective catheters, guidewires, and embolic coils, safe and effective closure of clinically significant PAVM shunt lesions can be accomplished. The purpose of this chapter is to review the topic of pulmonary arteriovenous malformations and percutaneous management of patients who have this disorder. Unlike acquired pulmonary arteriovenous (AV) fistulae, which may be secondary to metastatic disease, thoracic trauma, or long-standing cirrhosis, the more common congenital PAVMs arise from aberrant development of arteries and veins in a common capillary plexus.12 An abnormal connection develops between a pulmonary artery and vein branch, which bypasses the normal capillary bed and the process of oxygenation of blood. The PAVM shunt allows desaturated pulmonary arterial blood to pass into the systemic circulation via the pulmonary veins and left-sided heart chambers. The circulatory filtration by the lung capillary bed also is bypassed in the presence of the PAVM shunt, and thrombi or bacterial organisms in the venous system may pass through the shunt to the systemic arterial system (paradoxical emboli).13,14 Although they may go unrecognized at birth, these lesions enlarge over time, most likely because of low resistance to pulmonary blood flow, lack of normal wall musculature, and sustained pulmonary artery pressure. About two-thirds of lesions arise in the lower lobes, perhaps related to gravitational effects on the pulmonary artery blood flow with resulting increased hydrostatic pressure in the lower lobe pulmonary artery branches. The lesion architecture can be quite varied, but it usually consists of one or more dilated afferent arteries and efferent veins, between which lie a direct communication that can enlarge to form a saccular aneurysm (Fig. 11–1) or a convoluted, interconnecting network of vascular channels (Fig. 11–2).15 At the other extreme, lesion morphology is represented by multiple telangiectasias, which are small AV fistulae diffusely distributed in one or more lobes.16 Multiple large PAVMs also can occur, and a lobe of the lung may be largely replaced by the pathologic process. Pulmonary arteriovenous malformations create a right-to-left shunt between the pulmonary arterial and venous circulations. Experimental creation of such an AV fistula in dogs results in O2 desaturation of systemic arterial blood in relation to the size of the shunt.13 Cardiac output is increased in the form of increased stroke volume, and pulmonary vascular resistance, distal to the shunt, is decreased, most likely because of a compensatory vasodilation.13 Compensatory changes in stroke volumes and low pulmonary vascular resistance could explain why patients who have PAVM usually do not have associated cardiomegaly. FIGURE 11–1. Simple right lower lobe saccular PAVM. Injection in the proximal pulmonary artery shows a single segmental arterial supply to the malformation. FIGURE 11–2. A left lower lobe complex PAVM. This complex malformation has multiple segmental arterial supply. Oxygen content in the arterial blood of the PAVM patient will be low when a significant right-to-left shunt occurs. The degree of hypoxemia, however, may seem far more than anticipated compared with the size of the PAVM. Such disparity may be on the basis of numerous small AVMs, which may not be readily apparent angiographically, giving rise to a large total right-to-left shunt.17,18 It is often useful to quantify the magnitude of the right-to-left shunt, and several procedures are available. Shunt fractionation based on the O2 method is derived from data obtained with the patient breathing 100% oxygen. The shunt, as a fraction of total flow, is calculated from values of the oxygen content of idealized or nonshunted, arterial, and mixed venous blood.13,18,19 Because PAVM lesions tend to occur more often in the lower lobes, arterial oxygen content is lower when the patient is erect. This unusual positional phenomenon (orthodeoxia) is opposite to the normal physiologic state, in which O2 content may be unchanged or improved when the patient is erect.15,18 The PAVM patient will therefore drop O2 content when sitting or standing and experience a higher level of O2 content when supine. When dyspnea occurs, it may become more profound when the patient is erect (platydyspnea).19 Clinical manifestations of PAVM are usually dependent on the degree of right-to-left shunt. In addition to dyspnea, other signs of chronic hypoxemia, such as cyanosis and digital clubbing, may be present.20 Chronic hypoxemia also leads to the stimulation of erythropoiesis and development of polycythemia. The reticulocyte count and blood volume, on the basis of increased cellular volume, are higher than normal.21 PAVMs have a significant association with HHT, which is an autosomal dominant inherited disease characterized by systemic capillary dilatation and a bleeding diathesis.22–24 Over the past few years, the genetics of HHT have been further elucidated. Two distinct types of HHT have been identified: HHT type 1 is located on chromosome 13 with an associated mutation in the gene encoding endoglin (an endothelial cell protein). The locus for HHT type 2 was identified on chromosome 12, but the direct gene abnormality has yet to be understood. The phenotypes for both types 1 and 2 HHT are related to epistaxis and telangiectasia; however, there is a higher degree of pulmonary and cerebral involvement with type 1 HHT. The complete phenotype expression for type 2 HHT has not been fully explained.25–29 Clinical presentation with type 1 HHT is variable and dependent on the number of vascular lesions and the severity of organ dysfunction secondary to the lesions. Telangiectasias of the skin occur most frequently about the head, chest, and upper extremities. Involvement occurs in the gastrointestinal tract, lungs, brain, retina, and liver. Pulmonary lesions occur with a frequency of about 30% in patients with HHT, and patients diagnosed with PAVM have a high association with HHT (70%).30 The most common presenting manifestation in the respiratory tract in patients with HHT is epistaxis (78%). Almost one-third of patients have had a major bleeding episode that required transfusion, and more than one-half have developed epistaxis in the first decade of life.22 Orthodeoxia, which is decreased oxygen content in the erect position, can help establish the diagnosis of an underlying right to left shunt.23 Orthodeoxia in association with platyspnea can be nonspecific in its clinical presentation, however. Patients with this distinct sign and symptom complex have been recognized to have intracardiac shunts, pulmonary malformations, or pulmonary interstitial fibrosis.31 Dyspnea, cyanosis, and clubbing indicate significant hypoxemia in the patient with HHT. Patients with HHT may experience cardiac enlargement and high output failure, unlike patients with isolated PAVM,24 which may be on the basis of significant nonpulmonary shunts in the systemic circulation and iron deficiency anemia secondary to chronic blood loss in the gastrointestinal tract. The PAVM lesion shunts blood from the pulmonary artery to the pulmonary vein, bypassing the filtration effects of the pulmonary capillary bed. Patients with HHT and, as mentioned earlier, those with isolated PAVM, are at risk for paradoxical embolization from thrombi originating in the venous circulation or within the PAVM itself. Paradoxical emboli presumably account for central nervous system (CNS) manifestations of HHT, which may occur in up to 40 to 50% of patients with PAVM.32 Neurologic problems in the HHT patient include migraine headache (43%), transient ischemic attack (37%), a history of stroke (18%), and brain abscess (9%).30 The neurologic complications of stroke or brain abscess represent the most severe clinical manifestations of PAVM.32 The sudden onset of neurologic symptoms point to an embolic etiology; however, complicating hypoxemia, polycythemia, and coexistent cerebral AV fistulae also may play a role in their development. Brain abscess may have an embolic etiology, although cases of brain abscess have been reported in the absence of pulmonary lesions.33,34 In patients with HHT, gastrointestinal hemorrhage is the second most common presenting manifestation (44%), and up to 20% of patients require transfusion for major hemorrhage.1 Despite endoscopic techniques, the source of bleeding is often difficult to locate. Actively bleeding telangiectasias can be treated by electrocautery. Liver disease, as defined by hepatomegaly and transaminase elevations two times normal, has been reported in up to one-third of patients.1 Liver biopsy may show passive congestion, telangiectasia, and iron overload. A careful history and physical examination are essential in evaluating a patient with suspected HHT or PAVM. When epistaxis or telangiectasia or a first-degree relative with HHT occur in any combination, the diagnosis of HHT is established.30 In all patients with PAVM who are symptomatic from the lesion, the most frequent manifestations are dyspnea, easy fatigue, and cyanosis. As mentioned earlier, epistaxis is the most common clinical feature of HHT. Chronic hypoxemia will produce digital clubbing. On auscultation of the chest, a lung bruit may be heard. Measurement of arterial blood gases (ABG) will establish the degree of hypoxemia and can be obtained on room air with the patient in the supine and erect positions. Orthodeoxia occurs while the patient is in the erect position in a high percentage of patients with PAVM.18,35 Arterial blood gas values may be normal in some patients with solitary, isolated, and small PAVM lesions. A normal ABG level should not preclude further investigation in these patients. At the time of ABG determinations, shunt fractionation by the O2 method should be performed. With the patient breathing 100% oxygen for 15 minutes, O2 content can be used to quantify the magnitude of the right to-left shunt.13,19,30 These data are also useful as a baseline comparison to that obtained following embolization. Therefore, the effectiveness of therapy can be monitored by determining the residual shunt after embolization. Shunt fractionation is also useful in the long-term follow-up of patients in whom recanalization of an occluded PAVM or enlargement of small untreated lesions is a possibility. More recently, other methods of shunt determination have been reported.36–39 By measuring the fraction of an injected dose of technetiumlabeled albumin microspheres that reach the kidneys, the presence and quantification of a right-to-left shunt(s) can be measured.18 Qualitative shunt determination is also possible by using echocardiography microbubble techniques that rely on early recognition of microcavitation on an M-mode or two-dimensional image following injection of agitated saline.38,39 A high percentage of good-quality posteroanterior and lateral chest radiographs demonstrate the PAVM lesion. When the lesion is clearly demonstrated by chest radiography and ABG determinations are abnormal, the workup may proceed directly to pulmonary angiography.40 If the pulmonary angiogram fails to demonstrate a PAVM lesion with right and left main pulmonary artery injections of adequate contrast volume, the workup may be terminated. When the angiogram is positive, selective catheter injections in anteroposterior, oblique, and lateral projections will be necessary to delineate the angioarchitecture of the PAVM lesions.15 The role of computed tomography (CT) scanning in the diagnosis and follow-up of the patient with PAVM was evaluated by Remy et al.10 In their initial work in 1992, they examined 109 PAVMs in 40 patients using CT scanning and pulmonary angiography over 12 years, and they determined that CT scanning was superior to angiography in discovering small PAVMs. Lesions were detected 98% of the time by CT scanning, and an additional 38% were discovered by CT scanning that were not seen angiographically. In addition, CT scanning was better at detecting additional small feeding vessels not initially apparent on angiography and later detected after initiation of coil embolization of the large afferent arterial feeder. The significance of detecting secondary feeding vessels is that, if left unoccluded, they may enlarge and produce increasing hypoxemia over time. On postembolization CT scanning, the thin-walled aneurysms of PAVM lesions treated by embolotherapy were found to decrease in size in 47% of patients at 30 days following therapy, whereas 43% were unchanged, implying continuing patency. Later follow-up over a 4-year period on PAVM lesions treated with embolotherapy revealed that the aneurysm was no longer seen in 68% of lesions and detectable but decreased in size in 28%.10 With the advent of helical scanning technology, three-dimensional (3-D) reconstruction of pulmonary malformations is now possible. By combining data from both cross-sectional images and 3-D views, accurate evaluation of the angioarchitecture is possible. In 1994 Remy et al41 demonstrated that PAVM angioarchitecture could be analyzed confidently with 3-D views alone in 76% of malformations; however, by combining both the transaxial views with the 3-D analysis, more than 95% of the malformations could be classified correctly as either simple or complex malformations (Figs. 11–3 and 11–4). Thus, with the proper use of spiral CT, preoperative analysis of the angioarchitecture of PAVM can provide critical information to the interventionalist regarding the complexity of the malformation. FIGURE 11–3. Axial computed tomography of the chest demonstrating a single segmental arterial feeder to the malformation (Courtesy of Dr. L. Hofmann and Dr. E. Fishman.) FIGURE 11–4. Axial computed tomography of the chest demonstrating two separate segmental arterial feeders to this complex malformation (Courtesy of Drs. L Hofmann and E. Fishman.) Solitary case reports of magnetic resonance imaging (MRI) evaluations of pulmonary malformations are present in the literature, but the clinical utility of this modality has not been well established. Early studies reported that lesions are detectable and that image flow data could be useful in monitoring the effectiveness of therapy or recurrence of lesions.42 Before treatment, preoperative evaluation of pulmonary malformations should include extensive evaluation of the angioarchitecture of the lesion before embolization is done. Either with spiral CT or a diagnostic pulmonary angiogram, meticulous preembolization evaluation of the architecture of PAVM should be performed. Angiographic information of value to the interventional radiologist includes (1) the segmental location of the PAVM lesions; (2) the size, number, and location of the afferent pulmonary artery branches to the PAVM; (3) the nature of the shunt communication (i.e., simple aneurysm versus convoluted or vascular network); and (4) the presence of diffuse disease, which may not be amenable to embolotherapy. In 1983 White et al15 initially classifed PAVMs based on their angioarchitecture. Their classification was based on segmental pulmonary artery anatomy that was useful for embolotherapy. PAVMs were divided into simple and complex malformations. Simple malformations were defined as having a single segmental artery and draining vein. The complex type was defined as having two or more arteries that supply the PAVM with one or two draining veins. Within this classification, about 80% of all PAVMs were simple and 20% complex.15 Work with helical 3-D CT rendering of PAVMs, however, led to refinement of the classification by White et al.43 The simple malformation has been recognized to have one segmental arterial supply but may have one to three subsegmental arteries supplying the malformation (Fig. 11–5A). Usually selective injections demarcate the distal branching pattern seen within this type of malformation. Also, if multiple PAVMs arise from a single segmental artery, they are still classified as simple because they are supplied by a single segmental artery. Complex PAVMs are defined has having two or more different segmental arteries supplying the malformations (Fig. 11–5B). Interestingly, complex PAVMs are more common in the right middle lobe and lingula; White et al43 suggest that this may be related to additional sources of arterial supply from segmental basal branches.43

 Pathophysiology
Pathophysiology
 Hereditary Hemorrhagic Telangiectasia
Hereditary Hemorrhagic Telangiectasia
 Patient Evaluation
Patient Evaluation
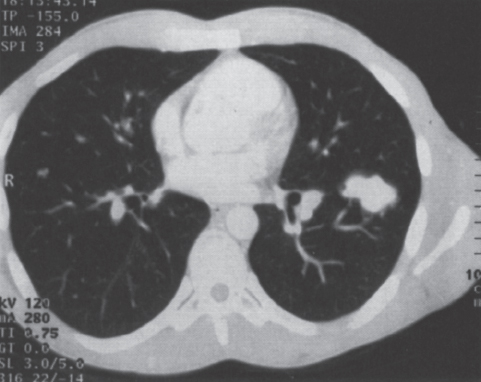
 Assessment of the Angioarchitecture
Assessment of the Angioarchitecture
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree