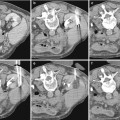
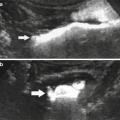
Fig. 1
(a) Catheter tip (arrow) seems correctly positioned, but renal artery opacification is faint for the prevalent opacification of celiac trunk and superior mesenteric artery, which overlays the right renal artery. (b) Selective renal angiography, a stenosis is present (arrow), as well as a poststenotic dilation (dashed arrow). (c) Renal arteries, correct visualization with a 20° LAO projection; a stenosis of the proximal left renal artery is evident (arrow)
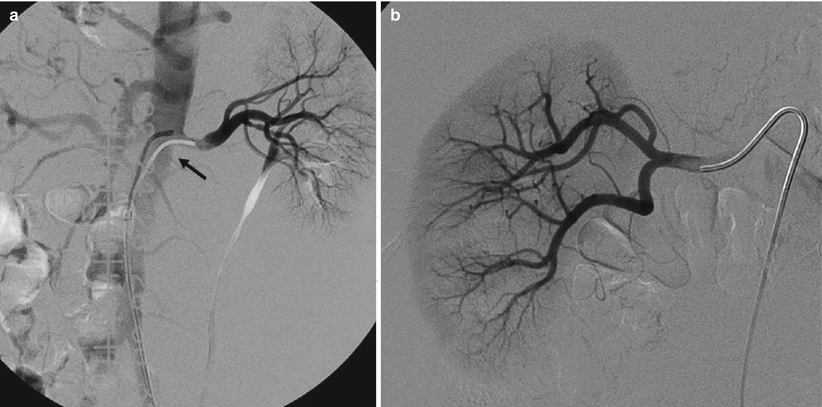
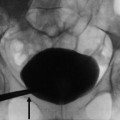
Fig. 2
(a) Selective renal angiography: an unstable catheter for shape and position can become dislodged (arrow) for the high flow during power injection. (b) If the catheter is positioned too distally in the main renal artery, the first part of the lumen is not visualized; an ostial stenosis could, therefore, go unnoticed
For acceptable diagnostic results, the equipment must be of high quality with X-ray tube and generator able to give good results in terms of spatial and contrast resolution also in corpulent patients. Modern DSA systems are based on digital fluoroscopy/fluorography systems and are equipped with special software and display facilities. The mask and the serial images are obtained from the television camera in analog form or from the charge-coupled device (CCD) television camera in digital form. Using a conventional TV camera with the image intensifier will require that the images are digitized in an analog-to-digital converter, which converts the TV line image (525/625 or 1,023/1,249 lines) to an image matrix, commonly with 512 × 512, 1,024 × 1,024, or 2,048 × 2,048 pixels. From a CCD camera, the images are digitized already in the electronics close to the light-sensitive CCD chip.
The last evolution of digital angiography systems is the introduction of flat-panel technology. This allows direct digital imaging without the intermediate step of analog-to-digital conversion with significant improvement of efficiency of the system. The prevalent method is based on an indirect X-ray conversion process, using cesium iodide scintillators. It offers considerable advantages in radiography, angiography, and fluoroscopy. The other method employs a direct converter such as selenium, which is particularly suitable for mammography. Both flat detector technologies are based on amorphous silicon active pixel matrices. Flat detectors facilitate the clinical workflow in radiographic rooms, with improved image quality, and provide the potential to reduce dose. This added value is based on their large dynamic range, their high sensitivity to X-rays, and the instant availability of the image. Advanced image processing is instrumental in these improvements and expands the range of conventional diagnostic methods. In angiography and fluoroscopy, the transition from image intensifiers to flat detectors is facilitated by the ample advantages they offer, such as distortion-free images, excellent coarse contrast, large dynamic range, and high X-ray sensitivity. Furthermore, another major advantage of flat-panel technology is that, although the tube is essentially unchanged, the image intensifier is eliminated. The image-receiving component of the unit, the flat panel itself, is smaller and more compact, allowing easier access to the patient during interventional procedures.
Another important facility in DSA systems is the postprocessing of images. The radiologist can use many processing options such as windowing, filtering, and quantitative measurements of distance and density. Sometimes, the anatomic structures move after the mask has been acquired, and for perfect subtraction, the mask has to be moved a few pixels or perhaps just a fraction of a pixel (pixel shift). Such functions are available in most DSA systems. The frame rate of acquisition must be quite high (at least 3 frames/s) in the first 3–5 s (arterial phase), then it can be decreased at 2 frames/s (parenchymal phase). In cases of prolonged acquisitions, when also a venous phase is acquired, the frame rate can be further reduced to 1 frame/s. Generally, when the diagnostic problem is focused on the arterial bed, only arterial and parenchymal phases are performed.
Concerning the position of the tip of the catheter, it should not be placed too cranial in order to avoid the filling of the celiac trunk and the superior mesenteric artery with contrast media, and, therefore, problems of overmatching of these arteries on the renal arteries (Fig. 1).
Considering the correct projections, it must be underlined that it is crucial to visualize the ostium of renal arteries, since it is the most frequent site of atheromatous lesions; their detection depends not only on their position but also on the size of the aorta, which once filled with contrast media can hide the origin of the renal arteries. In the second half of the 1990s, some papers were published on the contribution of CT elucidating this topic, based on the fact that CT is very effective in a precise evaluation of the position of the renal artery origin and their relationship with the aorta (Verschuyl et al. 1997a, b). These papers have shown that the left renal artery can be well depicted in the anteroposterior view because in 80 % of cases its origin is between 90° and 120° in respect to aorta axes, but it is useful to add a left anterior oblique (LAO) projection of 20°. Left renal artery shows a variable degree of origin and in 85 % of cases it is between −85 and −45°. For this side, the projection that better shows the right renal ostium is always the LAO at 20°, but sometimes an LAO at 40° is useful. In Verschuyl et al.’s experience (Verschuyl et al. 1997b), the routine use of AP and LAO projections at 20 and 40° gave a correct evaluation of 92 % of 400 renal arteries considered. In detail, the first projection to be done is the LAO at 20°, and if this one is not sufficient, the AP projection can be performed, followed by the LAO at 40° in selected cases. Other authors suggest doing only a LAO at 15° (Harrington et al. 1983).
Another alternative solution for renal artery evaluation is to proceed at selective angiography: this modality has the advantage to better define the distal branches of the main trunk; however, often it is unable to correctly evaluate the ostium because the tip of the catheter is placed distally with respect to the ostium Fig. 2).
It must be underlined that this type of maneuver is not totally risk-free, particularly in the case of atheromatous stenosis of the ostium for the risk of cholesterol embolization (Morita et al. 1989).
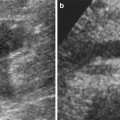
Another problematic issue is the detection of accessory or polar renal arteries, which are not an unusual event due to an incidence of 32 % in the monolateral form and 12 % in the bilateral form and may represent a cause of clinical problems and also technical problems (Verschuyl et al. 1997a). In some cases, these accessory arteries may originate on the same plane of the main trunk and, for this reason, reduce the detection of this vessel (Fig. 3). In these cases, oblique craniocaudal or caudo-cranial projections are useful to distinguish overlapping vessels and proceed to selective catheterization (Fig. 3b).
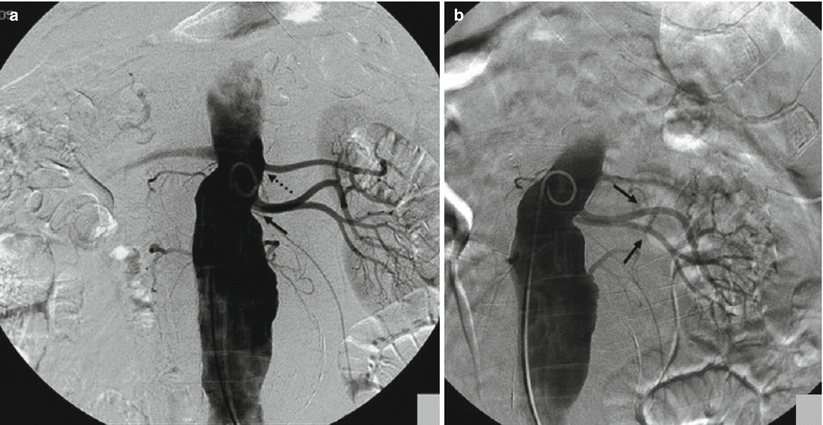

Fig. 3
(a) In the AP projection, the origin of the two accessory renal arteries (arrow) is on the same plane; the main trunk is more cranial (dashed arrow). (b) After craniocaudal projection, the origins of the two accessory renal arteries (arrows) are better defined
3 Interventional Renal Vascular Procedures
3.1 Vascular Interventions
Since its introduction in 1978, percutaneous transluminal renal angioplasty (PTRA) has emerged as a highly effective technique for the correction of renal artery stenoses. Renal angioplasty has notable physiologic, psychological, and economic advantages over other treatment modalities, and it should now be considered the therapy of choice for renovascular hypertension.
The indications of renal angioplasty are as follows (Kidney and Deutsch 1996):
Sudden onset of hypertension
Hypertension in a patient without a positive family history
Hypertension in a patient without a medical history of factors known to cause hypertension
Malignant hypertension
Hypertension refractory to pharmacotherapy
Patient noncompliance with medications
Hypertension in a patient with abdominal bruit suggestive of renal artery narrowing
Hypertension in a patient who develops renal failure while taking captopril
Sudden onset of hypertension in a young woman not taking oral contraceptives (for patients in this group, the likelihood of FMD is increased)
From a technical point of view, different modalities to perform endovascular treatment of renal artery stenosis are available as follows:
Simple balloon transluminal renal angioplasty, with eventual secondary stenting in case of suboptimal result at PTA
Primary renal artery stenting
Renal artery stenting with distal protection device
Regarding evolution of material employed in this type of intervention, the “trend” is to use materials derived from coronary intervention experience, and significant improvements have been introduced for diagnostic catheters, guidewires, PTA catheters, and stents.
Diagnostic Catheters: in the past, 4 or 5 French pre-shaped diagnostic catheters (Cobra, Renal Double Curve, Simmons, etc.) were used to catheterize a stenotic renal artery, and in association with hydrophilic guidewires, the stenosis was negotiated (Fig. 4a). Frequently, catheters were advanced beyond the stenosis and hydrophilic guidewires were changed with stiff Teflon-coated stainless steel wires (such as 0.035″ Rosen Heavy Duty), and standard profile PTA balloon catheters and premounted stents were advanced over this wire. In the last years, many centers have adopted the use of guiding catheters specifically designed for renal interventions. These guiding catheters, derived from coronary intervention experience, are larger in caliber (6–8 Fr) and have a large lumen. Usually they give a superior support compared to conventional diagnostic catheters and are advanced close to the ostium of the renal artery. At this point, the stenosis is negotiated with a 0.014″ or 0.018″ guidewire avoiding fragmentation of the atheromatous plaque. Then low-profile balloon catheter or premounted stents are advanced (Fig. 4b). This technique has been also referred to as the “do not touch technique” because it avoids, as much as possible, passages of materials and devices at the level of the atheromatous plaque.
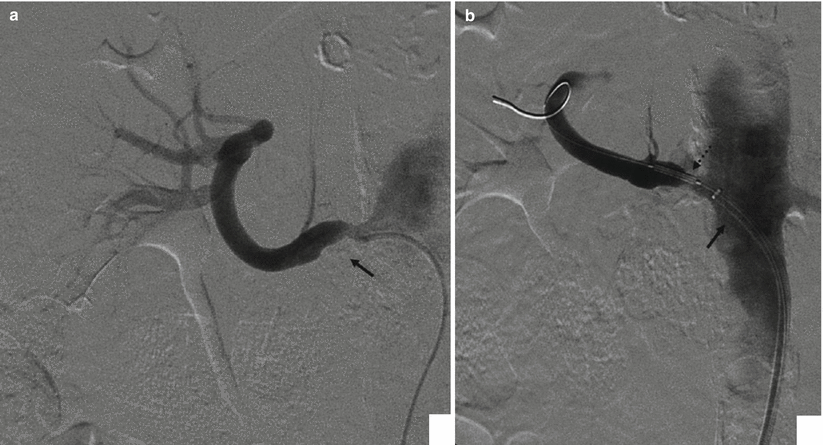

Fig. 4
(a) Selective renal angiography with a 5 Fr diagnostic catheter; a stenotic plaque (arrow) is present at the ostium. (b) Same patient, but angiography is now performed through the guiding catheter (arrow), during stent placement (dashed arrow). The advantage of a guiding catheter is that it enables to check every moment the different steps of the procedure
Guidewires: while negotiation of renal stenosis was done with 0.035″ guidewire in the past, several cath labs adopt the rule to negotiate stenosis with 0.014″ or 0.018″ guidewires in the last years, in order to decrease trauma to arterial plaques and therefore reduce distal embolization. Both stainless steel and nitinol guidewires are used, generally with a short soft tip, and are still able to give a support to the advancement of low-profile PTA balloon catheters or stents.
Balloon Catheters: for many years, standard balloon catheters were employed for the dilation of renal artery stenosis. These catheters have the following characteristics:
Shaft corresponding to a 5 Fr catheter.
“Over the wire” construction: this means that the entire catheter runs on the guidewire.
Short tip (shorter than 5 mm): this can be useful in the case of distal stenosis near the proximal division of the main trunk.
Balloon can be noncompliant or with low-compliance structure: in the first case, once the nominal pressure of the balloon (i.e., 8 atm) is reached, further increment of pressure will not lead to further increase in the diameter of the balloon. In the second case, once the nominal pressure of the balloon is reached, further increment of pressure will cause further smooth increase in the nominal diameter of the balloon till 10 % of the nominal diameter. The choice of the type of balloon is operator dependent, and well-defined guidelines do not exist.
In the last years, many centers have adopted the use of low-profile PTA balloon catheters derived from coronary interventions. These differ significantly from standard PTA catheters for the following reasons:
Very low-profile tip: the tip has a diameter of 2.6 Fr (0.66 mm) or lower compared to 1.65 mm of standard PTA balloon, and for this reason, an atraumatic advancement of the catheter through the stenosis is warranted.
Generally, this catheter is provided with a hydrophilic coating on the surface of the balloon and on the distal part of the shaft, which enables advancement.
Monorail or rapid exchange construction: this term defines a particular way of construction of the catheter for which only the last 30–40 cm of the catheters runs over the wire. Monorail systems give significant advantages because they avoid guidewire changes, avoid the use of long guidewires, and reduce procedural time.
Stents: also in the field of stents, significant evolutions have been introduced, moving from balloon expandable stent to be crimped over standard PTA balloon catheter to premounted balloon expandable stent on standard PTA catheter to the very last generation of low-profile premounted stent over rapid exchange or monorail balloon catheter. These last types of materials such as microguidewires and low-profile balloon catheters reduce the risk of traumatism to the atheromatous plaques responsible for distal embolization. Although in the past some authors treated renal artery stenosis with self-expandable stent (Raynaud et al. 1994), there is a general agreement that balloon expandable stents are much more preferable because their deployment is more precise and these stents have superior radial force. Until few years ago, only stainless steel stents were available, while in the recent years, new materials such as chromo-cobalt alloys have been introduced. This alloy has similar strength of stainless steel, but inferior weight and superior long-term results are expected, even if not proven currently. Few data are available on the use of drug-eluting stents in the treatment of renal artery stenosis. One recent multicenter trial in which a sirolimus-eluting stent was compared to a bare-metal stent shows that the angiographic outcome at 6 months did not reveal any significant difference between the two stents. Renal artery stenting with both stents significantly improved blood pressure. The paper concludes that only further studies with larger patient population and longer angiographic follow-up will be able to determine if there is a significant benefit of drug-eluting stents in treating ostial renal artery stenosis (Zahringer et al. 2007). Recently a paper (Lookstein et al. 2009) described a case of a patient who, after three failed revascularizations to keep the renal artery patent, underwent a sirolimus-eluting stent implantation which is still patent at the 24-month follow-up. Stents are mounted over noncompliant or low-compliant balloon catheters. In the first case, manufacturers offer stents with intermediate diameters such as 4.5, 5.5, and 6.5 mm, while in the case of stents premounted on low-compliant balloons, no intermediate measures are available due to the possibility of overdilation of these types of balloons. Generally, the lengths of the stents are 10, 12, 15, 18, and up to 24 mm. In addition, stents with a “flaring effect” are also available. This option allows the possibility to oversize the proximal diameter of the stent at the level of the ostium.
Postprocedural imaging has been used for follow-up and to assess the effectiveness of percutaneous revascularization, clinical criteria, and laboratory findings together. Serial Doppler ultrasound (US) is a readily available technique and able to diagnose residual stenosis or restenosis (Akan et al. 2003). Anyway, Doppler US is known to be challenging; even for the evaluation of native RAs owing to patient-related factors, its diagnostic performance in patients with implanted stents is additionally hampered by metal-induced waveform distortion (Sharafuddin et al. 2001; Parenti et al. 2008; Rocha-Singh et al. 2008). Magnetic resonance angiography after stent placement is limited by the ferromagnetic artifacts caused by most stents (Tello et al. 1998). More recently, technical advances with multidetector CT have pointed out with prospective studies (Steinwender et al. 2009) how CT angiography can provide excellent noninvasive technique to detect and evaluate intra-stent restenosis, in comparison with invasive DSA.
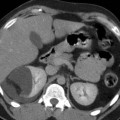
Distal embolic protection devices: this device was initially designed for carotid endovascular interventions as carotid artery stenting. It is a sort of microguidewire which mounts on the distal part a conic-shaped filter made of a membranous material such as polyurethane or nylon or a windsock-like nitinol basket, 2–3 cm distal to a floppy tip (Fig. 5). Distal protection devices work by interrupting or filtering blood flow in the internal carotid artery. Its use in renal interventions is still under investigation. Some centers have evaluated this tool on a significant number of cases (Henry et al. 2003). The most important characteristics are the ability to cross stenotic lesions and the “capture capability.” Generally, they are able to entrap embolic debris from medium to large size, generally particles more than 100 μm in long diameter. The aim of distal filter is to reduce embolization during the different phases of the maneuver. Some limitations in this filter exist due to the fact that they are not specifically designed for renal arteries, but for carotid arteries. The main limitation is that the “landing zone” of the filter may end distally to the main division of the main renal trunk and so only part of the kidney parenchyma is protected by the risk of debris embolization.
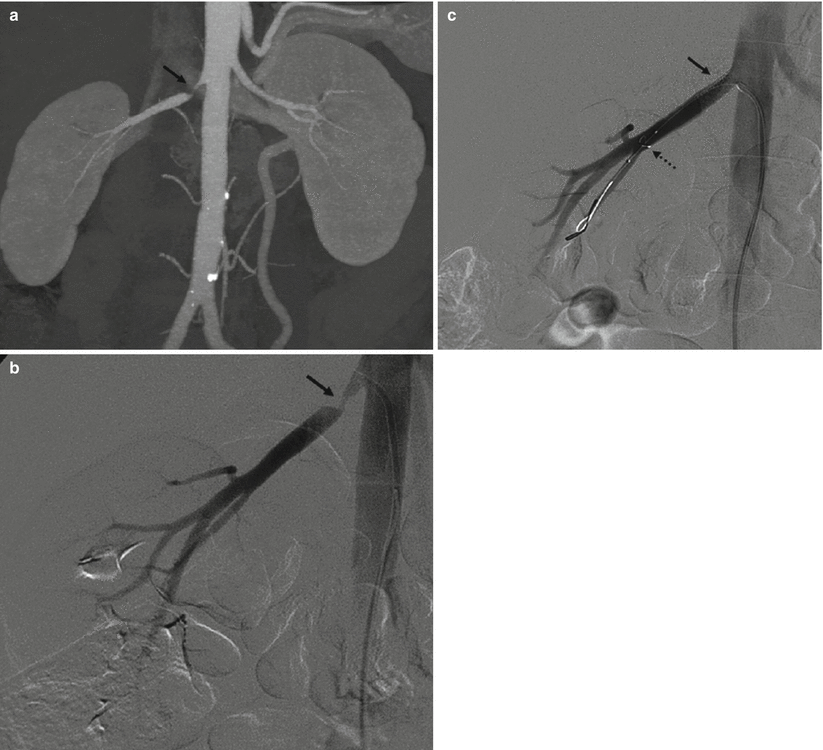

Fig. 5
(a) 3D MIP CT angio shows a high-grade short stenosis of the proximal right renal artery (arrow). (b) Preprocedural selective angiography confirms the lesion. (c) Postprocedural, a stent (arrow) has been placed and normal caliber has been restored. Note the embolic protection device in the distal segment of the main trunk (dashed arrow)
3.2 Other Endovascular Interventions
Arterial Embolization: renal conditions eligible to be occluded by means of an embolization procedure of a renal artery consist mainly of kidney tumors (both benign such as angiomyolipomas (AML) or malignant such as advanced renal cell carcinomas); the other common setting of embolic therapy is renal trauma.
From a technical point of view, this procedure needs confidence with the use of embolic materials such as:
Coils: these are mechanical devices with a helical shape variable in diameter and length. Frequently, nylon filaments are added on their surface. Coils can be pushable or detachable: the first ones, once released from the catheter, cannot be retrieved, while the detachable coils, if the final position is not correct, can be reintroduced in the catheter and changed with another coil. A recent evolution of coils is the so-called vascular plugs. They are mechanical devices made of nitinol mesh with different shapes, self-expandable, and able to achieve rapid occlusion of the target vessel. Once released in the vessel, if the deployment is not correct, the device can be recaptured and repositioned. When the plug is in the right position, it can be easily detached. Compared with the coils, it has the significant advantage that occlusion of the target vessel is obtained with a single device instead of several coils.
Particulate agents: most of them are based on polyvinyl alcohol (PVA) particles of different sizes from 50 to 1,200 μm. Usually, embolization with PVA particles causes a permanent occlusion of vessels of the size of the particles used. Recent evolution includes new types of microspheres consisting of a biocompatible acrylamide PVA macromer, which shows deformable capability and lower tendency to aggregate inside the catheter during injection with lower adverse body reactions. Alternatives are gelatine or fibrin sponge. These materials are manually reduced to small fragments by operator and then mixed with contrast media and slowly injected under fluoroscopic control. They are quite easy to use, but the main limitation is that the effect of this type of embolization is limited to a short period, and after 10–20 days, many of the vessels may be recanalized.
Liquid embolic agents: N-butyl-cyanoacrylate (NBCA) was the first liquid embolic agent applied in the clinical practice. It is a monomer acrylic glue which rapidly polymerizes when in contact with ionic media such as blood and causes a permanent occlusion. To avoid adherence to the tissue of the thin catheters required for the superselective embolization, NBCA has to be injected through a catheter washed with a 5 % dextrose solution and the catheter has to be withdrawn promptly after injection. The technique requires considerable expertise; there is also the risk of undesired embolizations. Moreover, NBCA polymerizes with an exothermic reaction, causing pain to the patient. Its use was mainly diffused in neuroradiologic embolization procedures, but in the last years, this product was abandoned also because it does not have the regular mark approval for an endovascular use (CE Mark). Glubran 2 is an acrylic glue bearing a CE mark authorized for surgical and endovascular use in neuroradiology and interventional radiology. The comonomer of Glubran 2 comprises a monomer of NBCA and a monomer of metacryloxysulpholane (MS) (owned by GEM Srl). MS allows the monomer of NBCA to polymerize with a lower exothermic reaction (45 °C) and a slightly longer polymerization time (Leonardi et al. 2002). Compared to the monomer NBCA, the Glubran 2 causes less pain to the patient and is associated with a lower risk of adherence of the catheter to the tissue, hence showing a greater ease of use. Differently, acrylic glues, once deposited into the nidus, determine its permanent occlusion and prevent its replenishment through feeding branches.
3.3 Renal Denervation
Renal denervation (RDN) is a new minimally invasive procedure for the treatment of refractory hypertension, based on the use of an endovascular catheter using radiofrequency (RF) ablation aimed at treating resistant hypertension. By applying RF pulses to the renal arteries, the nerves in the vascular wall (adventitia layer) can be denervated. This causes reduction of renal sympathetic afferent and efferent activity and blood pressure can be decreased (Esler et al. 2010). Early data from international clinical trials are promising, demonstrating average blood pressure reduction of approximately 30 mmHg at 3-year follow-up in patients with treatment-resistant hypertension (Symplicity HTN-1 2011; Esler et al. 2010).
From a historical point of view, before pharmacological management of hypertension, surgical sympathectomy was a recognized treatment for hypertension (Doumas and Douma 2009). This was often successful in reducing blood pressure, but unpredictable side effects of the procedure were poorly tolerated. Side effects included orthostatic hypotension, palpitations, anhidrosis, intestinal disturbances, loss of ejaculation, thoracic duct injuries, and atelectasis (Doumas et al. 2010).
Modern antihypertensive pharmacological interventions have improved the control of hypertension, but an estimated 30 % of hypertension cases are resistant. Resistant hypertension is defined as blood pressure above the target (140/90 mmHg) despite concomitant use of three or more antihypertensives – one of which should be a diuretic.
Several devices have been approved for this procedure. The first one is the Symplicity™ renal denervation system (Fig. 6a), produced by Medtronic (formerly Ardian), and consists of an endovascular catheter (6 Fr), RF generator, dispersive electrodes, foot switch, and power cable. The Symplicity device currently has over 5 years of clinical experience and 3 years of follow-up data. The Symplicity catheter is introduced using standard interventional technique via the femoral artery and is positioned in the renal artery under fluoroscopic guidance. The catheter is a 5 Fr compatible, single-use RF probe and it features a monopolar platinum electrode at the distal tip of the catheter that is used in conjunction with a standard dispersive electrode. The catheter is torquable, such that the user can easily rotate the catheter to treat different segments of the vessel. The treatment involves the delivery of relatively low-power and precisely focused RF bursts of approximately 8 W through the wall of the renal artery to disrupt the surrounding renal nerves lying in the adventitia. Multiple ablations must be done (6–8 for each renal artery) and the entire procedure may take from 40 to 60 min (Fig. 6b).
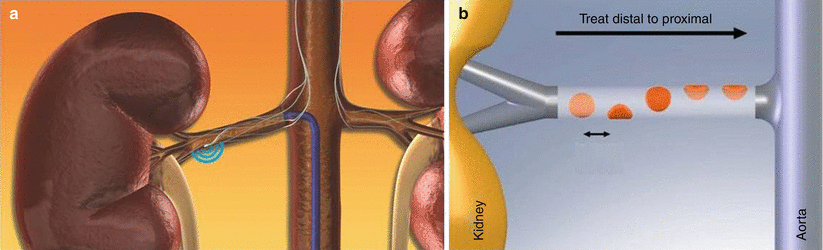

Fig. 6
(a) The probe is advanced in the right renal artery. RF is activated and sympathetic nervous fibers are disrupted. (b) Renal denervation plan of treatment. The probe is advanced distally in the renal artery, and multiple RF ablations are performed from distal to proximal, with an elliptical path. Both renal arteries must be treated
There are a number of other devices that are currently being evaluated, but these different technologies have limited clinical data and do not have long-term data. Some of these should have the advantage to do multiple ablations reducing the procedure time. In addition to Medtronic’s Symplicity system, other renal denervation devices have a CE mark. These include St. Jude Medical’s EnligHTN™ system, Vessix’s V2™ renal denervation system, Covidien’s OneShot™ system, and ReCor’s PARADISE™ system. Currently, no renal denervation device has FDA approval.
3.4 Renal Phlebography
From the diagnostic point, the only role left for renal phlebography is the sampling of blood from the renal veins in patients with renal hypertension to clarify which kidney is responsible for an increased production of the renin hormone. In these cases no particular imaging documentation of veins is required, unless anatomic significant variants are found. It is, however, important to obtain blood samples from each renal vein and from the inferior vena cava above and below the renal veins. Samples need not be simultaneous; however, it is better if they are closely spaced temporally. Renin levels from one kidney, which are >1.5 times of the contralateral kidney, are the indicative of renovascular hypertension. An increased renin value in the suprarenal inferior vena cava compared to the infrarenal vena cava is also suggestive of renovascular hypertension.
4 Anatomy and Variants
4.1 Arteries
In the majority of patients, the main renal arteries originate between the upper margin of L1 and the lower margin of L2 vertebrae, and frequently the origin of the right renal artery is higher than the left renal artery. The ostium of both renal arteries is located laterally; however, the ostium of the right renal artery tends to be located on the ventral aspect of the abdominal aorta, and as previously said and well shown on CT studies (Verschuyl et al. 1997a), there is a significant variation in its position.
The ostium of the left renal artery more frequently originates on the lateral aspect of the abdominal aorta; however, in a significant number of cases, it tends to be located on the dorsal wall of the aorta. The right renal artery in the first 1–2 cm has an anterior course and then turns posteriorly with a craniocaudal obliquity. The left renal artery has a more linear and horizontal course. The caliber of the artery in the proximal part generally is 6 mm. The main renal artery divides into segmental arteries near the renal hilum (Kadir and Brothers 1991). The first division is typically the posterior branch, which arises just before the renal hilum and passes posterior to the renal pelvis to supply a large portion of the blood flow to the posterior portion of the kidney. The main renal artery then continues before dividing into four anterior branches at the renal hilum: apical, upper, middle, and lower anterior segmental arteries. The apical and lower anterior segmental arteries supply the anterior and posterior surfaces of the upper and lower renal poles, respectively; the upper and middle segmental arteries supply the remainder of the anterior surface. The segmental arteries then course through the renal sinus and branch into the lobar arteries. Further divisions include the interlobar, arcuate, and interlobular arteries (Fig. 7).
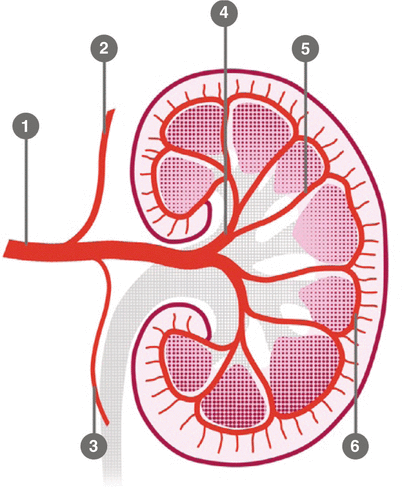

Fig. 7
Schematic anatomy of the renal arteries and branching: 1 main trunk, 2 adrenal artery, 3 gonadal artery, 4 segmentary artery, 5 lobar artery, 6 arcuate artery
Renal artery variations are divided into two groups: early division and extrarenal arteries. The branching of the main renal arteries into segmental branches more proximally than the renal hilus level is termed as early division (Fig. 8), while the “extrarenal artery” is divided into two groups: hilar (accessory) and polar (aberrant) arteries. The hilar arteries enter the kidneys from the hilum with the main renal artery, whereas the polar arteries enter the kidneys directly from the capsule outside the hilum. The origin of these extra renal arteries is quite variable, and the majority of them arise from the abdominal aorta over or below the main trunk. Infrequently, they may arise from the common iliac arteries. Renal artery origins arising above the superior mesenteric artery are extremely rare. Congenital anomalies of renal position and conformation are often associated with aberrant location of renal artery origins and supernumerary vessels. In particular, horseshoe kidney has a 100 % incidence of multiple renal arteries (Fig. 9). The renal pelvis and proximal ureters are supplied by small branches of the interlobular, arcuate, and distal main renal arteries. The middle portion of the ureters is supplied by the gonadal arteries (Fig. 7). The distal ureters are supplied by terminal branches of the internal iliac arteries, most notably the cystic artery.
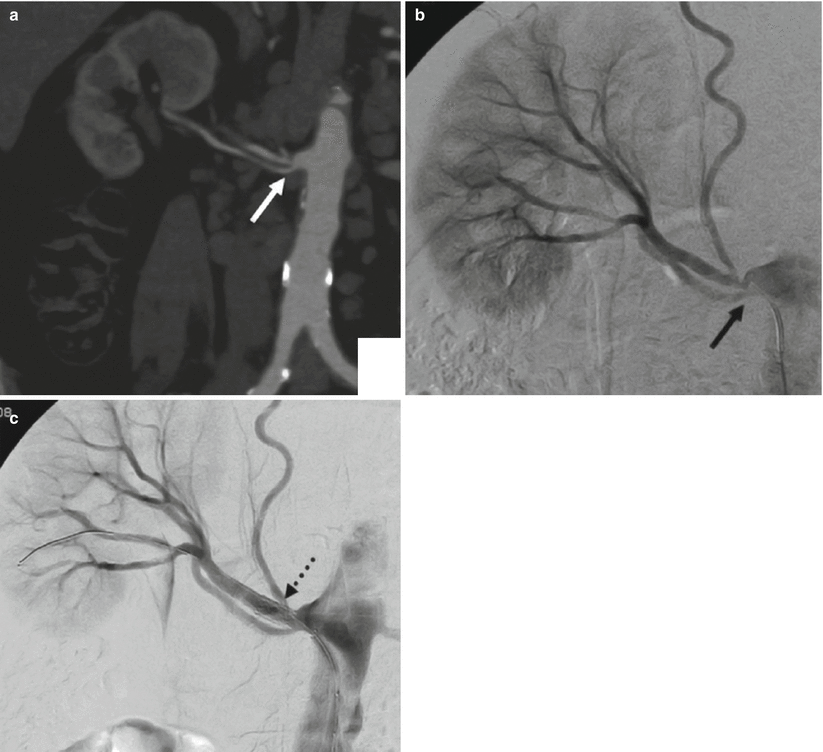
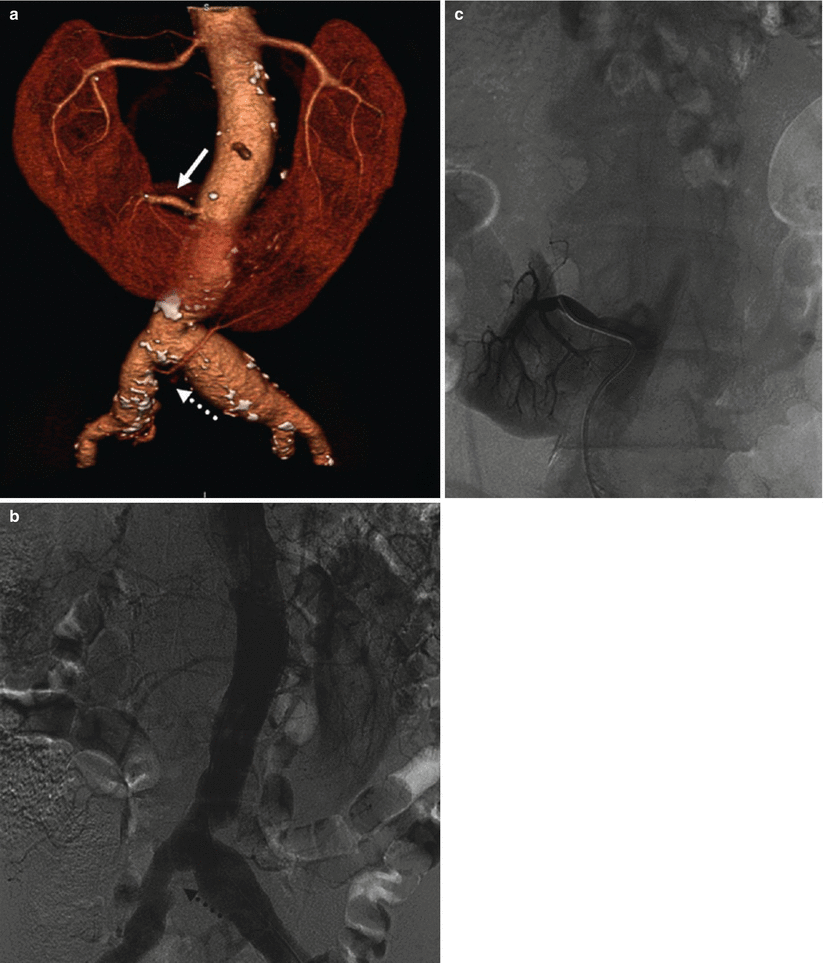

Fig. 8
(a) CT angio shows an early division (arrow) from the main renal trunk, with an eccentric stenosis of the superior artery. (b) Same patient, selective angiography confirms the finding. (c) Angiography poststent deployment (dashed arrow)

Fig. 9
(a) CT angio shows a horseshoe kidney in a patient with an abdominal aortic aneurysm involving both iliac arteries. Note the two polar arteries, the first (arrow) arising from the abdominal aorta and supplying the right inferior renal pole and the second (dashed arrow) arising from the proximal tract of the right common iliac artery and supplying the left lower renal pole. (b) Diagnostic aortography failed to show the polar arteries; only the second (dashed arrow) is barely visible. (c) The first polar artery is later shown after selective catheterism
In several anatomic and angiographic studies, it was stated that the rate of bilateral extrarenal arteries is 10–15 % (Kadir and Brothers 1991; Talovic et al. 2004), that the rate of early division in the general population is 15 %, that aberrant renal arteries are observed twice as often as accessory renal arteries and the frequency rate of extrarenal arteries is the same on the right and left side, and that in 12 % of the general population, extrarenal arteries are bilateral. In a recent paper (Ozkan et al. 2006), the frequency rate of early division and bilateral extrarenal arteries is 8 and 5 %, respectively, which is low when compared to other major series. In this series, no statistically significant differences were detected between the frequency rates of ERA on the right and left side, and this finding was compatible with other studies.
The renal arteries are end arteries. In contrast to other districts (i.e., the colonic and hepatic), the intrarenal collateral pathways are poorly developed. In the presence of slowly progressive proximal renal artery stenosis, renal capsular, ureteral, adrenal, and other retroperitoneal arteries may enlarge sufficiently to provide a collateral blood supply to keep kidney perfusion, but with a compromised function. Acute proximal occlusion of a previously normal renal artery results in profound ischemia due to insufficient preexisting collateral supply.
4.2 Veins
The renal cortex is drained sequentially by the arcuate veins and interlobar veins. The lobar veins join to form the main renal vein. The renal vein usually lies anterior to the renal artery at the renal hilum. The left renal vein is almost three times longer than the right renal vein. The left renal vein averages 6–10 cm in length and normally courses anteriorly between the superior mesenteric artery and the aorta before emptying into the medial aspect of the inferior vena cava. The right renal vein averages 2–4 cm in length and joins the lateral aspect of the inferior vena cava. Unlike the right renal vein, the left renal vein receives several tributaries before joining the inferior vena cava. It receives the left adrenal vein superiorly, the left gonadal vein inferiorly, and a lumbar vein posteriorly.
While anatomic variants of the renal veins are commonly visible in CT, their demonstration during renal phlebography is not so obvious and easy. Multiple renal veins constitute the most common venous variant and are seen in approximately 15–30 % of patients (Kadir and Brothers 1991). Multiple right renal veins occur in up to 30 % of individuals, and sometimes a single vein may divide before joining the inferior vena cava (Beckmann and Abrams 1980).
The most common anomaly of the left renal venous system is the circumaortic renal vein, seen in up to 17 % of patients (Kahn 1973; Ferris 1969). In this anomaly, the left renal vein bifurcates into ventral and dorsal limbs that encircle the abdominal aorta. The posterior limb is usually the smaller of the two, although this is certainly variable. There are two common variants of the circumaortic vein: in the most frequent (approximately 75 % of cases), one renal vein at the renal hilum subsequently divides before entering the inferior vena cava; in the less common variant, two distinct veins originate from the renal hilum (Beckmann and Abrams 1979, 1980). When a circumaortic renal vein is observed, the gonadal vein will typically join the retroaortic limb and the adrenal vein will join the preaortic limb (Kadir and Brothers 1991). A less common venous anomaly is the complete retroaortic renal vein, seen in 3 % of patients. Here, the single left renal vein courses posterior to the aorta and drains into the lower lumbar portion of the inferior vena cava. Alternatively, the retroaortic renal vein can drain into the iliac vein (Satyapal et al. 1999).
5 Occlusive Disease
The majority of renal occlusive disease (>90 %) is due to atherosclerosis and FMD. These pathologies can clinically be asymptomatic or arise with symptoms and signs, consisting of arterial hypertension or renal failure. Less common causes of stenosis of the renal artery are dissection, vasculitis, neurofibromatosis (NF), and compression from mass effect (i.e., neoplasia, hematoma), and developmental abnormalities.
The majority of hypertensive patients (95 %) are affected from what is referred to as primary hypertension; in other words, there is no structural abnormality that can be isolated as the cause of the condition. In only 1–5 % of the hypertensive population, the condition can be attributed to the stenosis of renal arteries; in that case it is then classified as secondary.
The mechanisms of arterial hypertension due to stenotic disease of the renal artery are based on the activation of the renin-angiotensin-aldosterone system (RAAS) (Fig. 10).
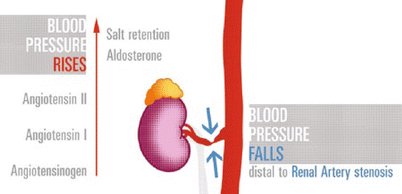

Fig. 10
RAS cascade. Critical stenosis at the main renal artery reduces flow and pressure distal to it. Kidney releases renin (not shown) that converts angiotensinogen to angiotensin-I (AT-I), then metabolized into the lungs, to angiotensin-II; the effects are constriction of arterioles and rising of the aldosterone level. The final outcome is rise in systemic blood pressure
In the human physiology, the most important mechanism to control circulatory volume on the long term is the renal-body fluid feedback system. If the arterial pressure is too high, the kidney excretes increased quantities of sodium and water, and on the other hand, when arterial pressure falls, the kidney reduces the rate of sodium and water excretion until pressure returns to normal levels. This mechanism acts over the period of several days.
The kidneys control pressure through the renin-angiotensin-aldosterone system (RAS). When the arterial pressure falls too low, the kidney releases a small protein (renin) that activates the RAS. This helps to raise arterial pressure in several ways and by doing so corrects the initial fall of pressure. Angiotensin-II (AT-II) is the end product of this chain and acts by constricting the arterioles and veins throughout the body, raising the total peripheral resistances and decreasing the vascular capacity (Guyton and Hall 2006).
This consideration explains how stenosis of renal arteries causes hypertension. This behavior has been described in the “two-kidney one-clip” hypertension model by Goldblatt (Goldblatt 1934; Glodny and Glodny 2006). In this experiment, a constrictor is placed on a renal artery of an animal; thus, the reduced blood flow to the kidney induces the production of large amounts of renin from the cells of the juxtaglomerular apparatus. For several days, there is a steady increase in blood pressure until the arterial pressure rises to a level that allows return to normal renal perfusion. The same happens in clinical renovascular hypertension.
About the clinical presentation, there are some signs that are characteristic of renovascular hypertension compared to the primary form. Generally, renovascular hypertension is hard to control with drug therapy; moreover, the onset and the increase in blood pressure are usually sudden; in addition, during physical examination, a high-pitched epigastric bruit can be present. As previously stated, patients are poorly responsive to drug therapy; moreover, if treated with ACE inhibitors or AT-II blockers, acute renal failure may appear, with consequent rise in serum creatinine.
Further manifestations of renal hypertension are left ventricular dysfunction and renal dysfunction. The first is due to left ventricular hypertrophy as a consequence of myocardial fibrosis and leads to diastolic and later systolic dysfunction with significant morbidity and mortality. The second one, renal dysfunction, may be caused by severe bilateral renal artery stenosis or functional single kidney. An important role may have been the contemporary presence of diabetes mellitus causing nephrosclerosis.
Duplex Doppler ultrasonography is a good screening test in many patients but has limitations in larger persons and can overlook small accessory arteries. For patients with normal renal function but a high clinical index of suspicion for renovascular disease, contrast-enhanced magnetic resonance angiography and computed tomographic angiography are the most accurate imaging tests (Hartman and Kawashima 2009) (Table 7.1).
Table 7.1
Clinical presentation of vascular ischemic renal disease
Acute renal failure (ARF) |
Progressive azotemia in a patient diagnosed with renovascular hypertension |
New onset of azotemia in a patient without a history of renal hypertension |
Hypertension and azotemia in a renal transplant patient |
5.1 Atherosclerotic Renal Artery
Most of the patients with a positive imaging for renal artery stenosis are asymptomatic. At angiography, atherosclerotic plaques are usually localized within 1 cm to the ostium, more often consisting of stenosis at the origin, since less than 10 % of the stenosis are distal than 1 cm. On that basis, the lesions can be classified as ostial stenosis or proximal stenosis, and on the other hand, intrarenal branches are affected on a variable degree. Frequently, plaque formation begins in the aorta wall with progression into the renal artery lumen, inducing the typical appearance of the ostial, usually eccentric, location of the atherosclerotic renal artery stenosis.
The epidemiology of atherosclerotic plaques is focused on male rather than female gender, affecting especially individuals older than 60 years. In a large autopsy series, stenosis producing more than >50 % of reduction in caliber was found in 18 % of those between 65 and 74 years and in 42 % of those older than 75 years (Fauci and Harrison 2009). The quantification of a renal stenosis on angiography is based on the degree of luminal narrowing: some authors consider significant a narrowing superior or equal to 50 %, while other authors tend to set a 70 % degree of stenosis as the threshold (Olin et al. 1995) (Fig. 11




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree