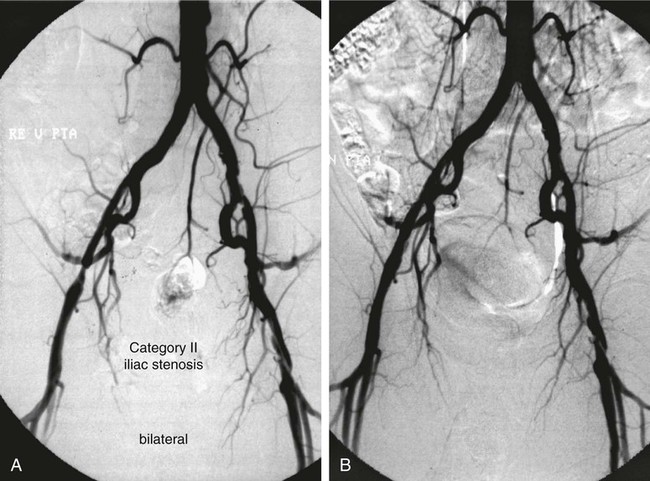
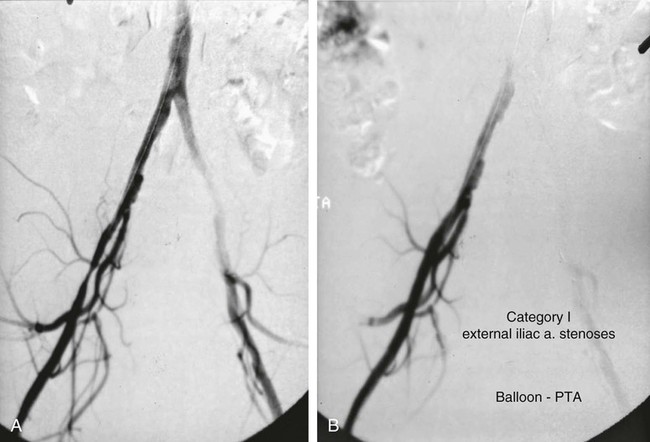
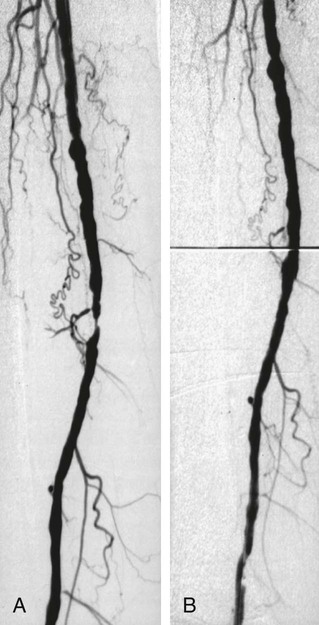
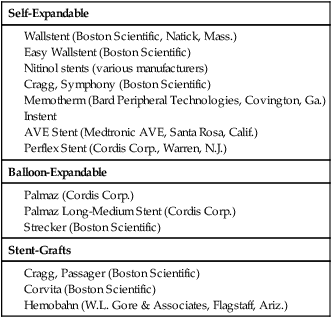
Shawn N. Sarin, Eberhard Zeitler*, Reinhard Loose, Prasanna Vasudevan and Anthony C. Venbrux Since its introduction, PTA (with and without stenting) has become standard practice in all arteries of the extremities, as well as in other areas of the body, including the coronary1,2 and carotid3 circulations. The increasing success of angioplasty of the coronary arteries led to general clinical acceptance of angioplasty as an alternative to open surgery. Subsequent technical developments and the introduction of additional antiplatelet aggregation drugs helped improve clinical results. There are basically two types of stent configurations: balloon-expandable (BX) and self-expandable (SX) stents. They are constructed of different materials and have different designs (Table 25-1). To reduce the problem of in-stent restenosis, drug-eluting stents and bioabsorbable stents are now being evaluated in clinical trials. At present, PTA without stents is less costly than PTA with stents, so stent use requires specific clinical indications derived from results of randomized trials. Use of drug-eluting stents is safe for acute complications, but for elective applications, results from randomized prospective trials are necessary. TABLE 25-1 The incidence of peripheral occlusive vascular disease (POVD) varies between 2.7% and 4.0%, without regard to differences in age and sex. Between the ages of 70 and 79 years, the prevalence of POVD is 9.8% in men and 7.7% in women. Coronary artery disease (CAD) is 2.5 times more frequent in patients with POVD than in individuals with healthy peripheral arteries. In contrast, POVD develops 2.3 times more often in patients with CAD than in persons without coronary symptoms; a strong correlation exists between CAD and POVD.4,5 The prognosis for POVD if untreated is poor, with about 25% of patients dying within 5 years and 17% requiring leg amputation. Follow-up studies of patients with intermittent claudication (Rutherford classification 2 and 3) show that 50% die within 10 years, 20% do not change, and 20% experience symptom deterioration. Clinically, only 10% improve spontaneously.6 Results can be optimized with the combination of open surgery and endovascular treatment. The original TASC classification gives recommendations for the management of POVD secondary to atherosclerosis affecting the lower limbs and seeks to aid physicians in selecting a suitable treatment. TASC II (2007),7 more recently released, reflects the evolution of the preferred options for treating femoropopliteal lesions. Most diagnostic and interventional angiography systems are designed as a “C-arm” or a “U-arm” so the equipment can be moved around the patient easily to acquire multidirectional imaging projections during dynamic fluoroscopic imaging without subtraction or during digital subtraction angiography. If possible, the systems should be designed with the x-ray tube under the table to reduce occupational exposure. If horizontal or oblique projections are necessary, the physician should always stand at the detector side of the patient and not at the tube side. Additional and mandatory options for reduction of patient and occupational exposure include last image hold, a second monitor for reference images, pulsed fluoroscopy, and virtual collimation without radiation.8 In 2002, the first dynamic flat-panel detectors were introduced, replacing the image intensifier. These detectors are now available with dimensions up to 40 × 40 cm. When compared with image intensifier systems, they provide higher spatial resolution (up to 3.25 line pairs per millimeter), homogeneous signal intensity over the entire image, no geometric distortion, and a better signal-to-noise ratio with a smaller dose of radiation.9 In 2004 the first flat-panel detector computed tomography (CT) systems were introduced for clinical use. With a single rotation for unsubtracted images or two rotations (forward/backward) for subtracted images, up to 2000 thin CT slices can be reconstructed. The time needed for a 240-degree rotation ranges from 5 to 20 seconds, and the dose to the patient does not exceed that of a conventional multislice CT.10 Descriptions of normal arterial anatomy of the abdominal aorta, pelvic arteries, and upper and lower extremities are derived from several standard texts and included elsewhere in this book. Knowledge of vascular variations and sites for percutaneous introduction of catheters into the arterial system is important for performing interventional techniques.11,12 The SFA is a unique vessel in its anatomy, function, and interventional requirements. It is not comparable to any other arterial vascular bed. The SFA is a long vessel with high resistance to flow in varied hemodynamic conditions. In the past, the unique characteristics of the SFA have resulted in suboptimal outcomes for specific endovascular procedures. Although some endovascular techniques used in the SFA have been studied in prospective randomized trials, the results have been disappointing. Five-year long-term patency rates ranging between 50% and 60% have been reported. This is lower than rates reported in other arterial vascular beds.13 Angioplasty for treating arterial stenosis or obstruction may be divided into steps: 1. Pretreatment angiography with localization of the arterial obstruction 2. Crossing the lesion with a guidewire or catheter with a flexible tip 3. Advancement of the treating catheter or instrument over the guidewire and confirming patency of runoff arteries 4. Exchange of the diagnostic catheter for the balloon catheter or stent 5. Dilation of the stenosis with the angioplasty balloon, followed by deflation 6. Completion angiography followed by treatment of runoff vessels with different balloon catheters or stents 7. Exchange of catheter materials and occlusion of the arterial puncture site by manual compression or a closure device 8. Placement of a compression bandage above the puncture site, followed by patient monitoring for a minimum of 2 hours 9. Depending on the patient’s clinical situation and condition and the outcome of the angioplasty procedure, monitoring the patient for several more hours 10. After discharge, if there is no contraindication, patients may be treated for 2 more days with medications such as anticoagulant or antiplatelet drugs. Modification of risk factors is also important. In summary, the pathomorphologic mechanism of angioplasty can be described as a “controlled traumatic injury” that leads to dilation, with a free arterial lumen. This effect can be verified by histologic and angiographic examination and is summarized in Table 25-2. TABLE 25-2 Mechanisms of Percutaneous Transluminal Angioplasty* *There is a risk of distal embolization and vessel rupture (infrequent). Angioplasty with balloon catheters as originally described by Gruentzig is successful in treating single stenoses of the common and external iliac arteries (Figs. 25-1 to 25-3) via a retrograde transfemoral approach. To treat stenoses in the groin region, the CFA of the contralateral side is punctured, and treatment is accomplished as described earlier (i.e., “up and over” the iliac bifurcation). In patients with stenosis of the infrarenal aorta or occlusion of the iliac arteries on both sides (see Fig. 25-2), bilateral retrograde femoral artery puncture is required. To dilate stenoses close to the aortic bifurcation or in the aorta itself, simultaneous dilation with two balloons (i.e., the kissing balloon technique) or a balloon with a large diameter is necessary. Dilation with three balloons has been reported in the literature (i.e., balloon catheters inserted from the groin puncture sites and one from the brachial artery directed downstream [i.e., caudally]). Indications for the different types of treatment of POVD in the aortoiliac segment are summarized in the TASC 20077 document, which states that treatment of type C lesions can be endovascular or surgical. If endovascular techniques and conventional surgery have comparable short- and long-term results, the technique associated with the least morbidity and mortality is preferred. In patients with claudication, more often single (Figs. 25-4 and 25-5) or multiple stenoses can be crossed safely with the guidewire and catheter. In some patients, the diagnostic catheter is exchanged for the balloon catheter. In others, after carefully advancing the guidewire, the balloon catheter can be introduced directly over the guidewire in situ.
Angioplasty
Stenting
Self-Expandable
Balloon-Expandable
Stent-Grafts
Clinical Relevance
Global Prevalence of Peripheral Occlusive Vascular Disease
Equipment
Technique
Anatomy and Approach
Technical Aspects

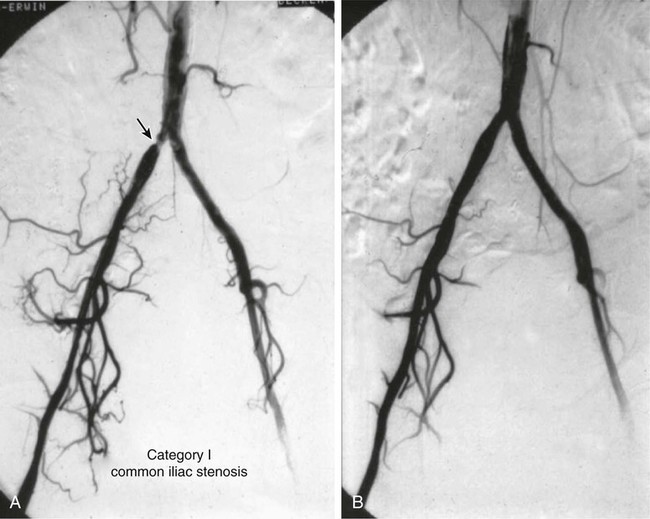
Angioplasty for Occlusion of the Iliac Artery
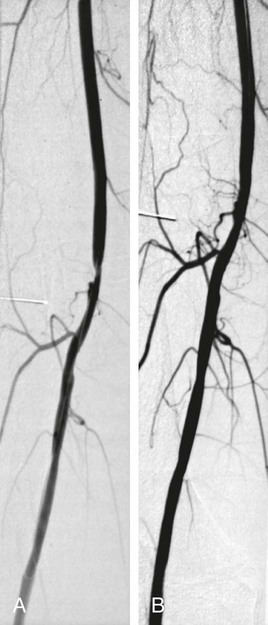
Angioplasty of the Superficial Femoral and Popliteal and Tibioperoneal Arteries
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Angioplasty