Chapter Outline
Splenic Clefts, Lobulations, and Bands
Embryology
Splenic development begins in the fifth week of gestation from a condensation of mesenchymal cells that aggregate between the two leaves of the dorsal mesogastrium. Aggregates from several adjoining areas fuse to form a lobulated embryonic spleen. Rotation of the stomach and growth of the dorsal mesogastrium cause the translocation of the developing spleen from the midline to the upper left side of the abdominal cavity, and the mesogastrium fuses with the peritoneum over the left kidney, forming the splenorenal ligament ( Fig. 104-1 ). This causes the lienorenal ligament to fuse dorsally, and the splenic artery has a course to the left behind the peritoneum as it enters the splenorenal ligament in the adult.

The capsule, connective tissue framework, and parenchyma of the spleen form from differentiated mesenchymal cells. The spleen serves a hematopoietic function from the fourth to the eighth month of gestation. Lymphocyte and monocyte production, however, continues throughout life.
Splenic Clefts, Lobulations, and Bands
In the fetal period, the spleen is lobulated; however, the lobulations normally disappear before birth. Lobulation may persist along the medial (hilar) aspect of the spleen and is considered a normal feature of splenic anatomy. Notches or clefts along the superior border are remnants of the grooves that originally separated fetal lobules. An abnormally deep fissure may give the appearance of a band. If the fissure traverses the entire spleen, a waist is created that may mimic a laceration. Peritoneum may be embedded on this waist and may mimic a hematoma, infarction, or laceration. Deep fissures tend to occur along the superior border of the spleen. When they occur along the inferior border, a lobule of splenic tissue may extend medially and anteriorly to the left kidney. Less frequently, the lobule may lie posterior to the upper pole of the left kidney and displace it anteriorly.
Wandering or Ectopic Spleen
Wandering or ectopic spleen refers to migration of the spleen from its normal fixed posterolateral location in the left upper abdomen. The reported incidence is 0.16% on the basis of 3853 splenectomies performed for various indications involving all ages. It usually is manifested between the ages of 20 and 40 years; 70% to 80% of reported cases are in women in the reproductive age. One third of cases are found in children, most (70%) older than 10 years.
The spleen is normally anchored in the left upper abdomen by two ligaments: the gastrosplenic ligament, which connects the spleen to the greater curvature of the stomach; and the splenorenal ligament, which connects the spleen to the left kidney and the posterior body wall.
The etiology of wandering spleen may be congenital or acquired. Congenitally, the condition is from absence or weakness of one or more of the ligaments that affix the spleen in its normal position in the upper left abdomen. The spleen is instead solely attached by its vascular pedicle and can become a wholly intraperitoneal hypermobile organ. Rarely, a normal spleen may be seen in the left upper quadrant, and an accessory spleen may be mobile and wander.
The pathologic process also may be acquired as a result of splenomegaly or ligamentous laxity, such as in pregnancy or muscular dystrophies. There is a higher incidence of wandering spleen in multiparous women, which suggests an etiologic role of hormonal effects and abdominal laxity associated with pregnancy. Wandering spleen has been reported in prune-belly syndrome and in cases of diseases causing splenic enlargement, such as splenic cysts, malaria, Hodgkin’s disease, and lymphangioma.
Clinical Findings
The clinical presentation of wandering spleen is variable. Affected patients may be asymptomatic and the condition may be detected incidentally. Diagnosis may be made incidentally as a mass on physical examination or imaging. Classically, a firm, movable, notched mass can be palpated. Symptoms occur when there is pressure on the vascular pedicle of the spleen or when torsion develops.
The most common presentation in children is acute abdominal pain. Patients may also present clinically with nonspecific symptoms, such as occasional nausea, emesis, or mild, crampy abdominal pain. Pain may be vague in cases of chronic intermittent splenic torsion. Wandering spleen with torsion may involve the tail of the pancreas, resulting in pancreatitis, and the splenic venous occlusion may result in splenomegaly and cause hypersplenism. Less common presentations of wandering spleen include small bowel or colonic obstruction and even duodenal obstruction due to compression by the splenic pedicle.
Radiologic Findings
Conventional Radiography
Supine and erect abdominal radiographs may show absence of the normal splenic silhouette or a mobile mass in the left or central abdomen. With the spleen absent from its normal location, bowel loops may fill the splenic fossa. The left kidney may be elevated and lack the usual splenic impression or hump and may cause elevation of the left hemidiaphragm.
With the spleen not fixed in the abdomen, the elongated pedicle of the spleen or the displaced spleen can cause gastric outlet obstruction, small bowel obstruction, or colonic obstruction. Abdominal radiographs can show a distended stomach, a small bowel obstruction pattern, or colonic obstruction. The splenic pedicle may cause a linear defect across the involved bowel segment or colon.
Nuclear Scintigraphy
Technetium Tc 99m sulfur colloid scintigraphy can identify reticuloendothelial activity in the liver as well as in the spleen and is useful in identifying ectopic functional splenic tissue in the abdomen. Similarly, heat-denatured 99m Tc-labeled red blood cell imaging, specific for splenic tissue, provides useful information for spleen size and localization as well as for the specific red blood cell sequestration function of the splenic tissue. A wandering spleen is diagnosed by normal uptake of radiotracer by functioning splenic tissue in an abnormal location in the abdomen ( Fig. 104-2 ). The lack of tracer activity in a previously demonstrated wandering spleen indicates torsion.

Ultrasound
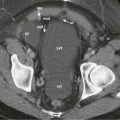
Gray-scale sonography and power Doppler sonography are valuable in the diagnosis of wandering spleen and allow the diagnosis of complications such as torsion and infarction. Sonography can confirm the absence of the spleen in its normal position in the left upper abdomen and can identify the ectopic spleen in the abdomen or pelvis ( Fig. 104-3 ). The ectopic spleen is of uniform echotexture with a peripheral indentation on the mass, representing the hilar vasculature. Use of power Doppler, color Doppler, and duplex sonography allows evaluation of blood flow in the splenic parenchyma and in the major splenic vessels.

Real-time sonography can be used to identify splenic hypermobility and twisting of the vascular pedicle, which can increase risk of infarction. The spleen may be enlarged owing to an intermittent twist of the vascular pedicle and venous congestion.
Computed Tomography and Magnetic Resonance Imaging
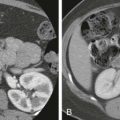
Contrast-enhanced computed tomography (CT) can diagnose an ectopic spleen and its associated complications and is often performed as patients may present with abdominal pain. A pathognomonic CT finding of a wandering spleen is the absence of the spleen in the upper abdomen and the presence of a well-marginated homogeneously enhancing soft tissue mass in the abdomen or pelvis ( Fig. 104-4 ).

Features of splenic torsion include poor or absent enhancement of splenic parenchyma, hyperdense pedicle indicating thrombosis, and enhancing splenic capsule from peripheral circulation. The whirl appearance of the splenic vascular pedicle is a specific sign of torsion. Splenomegaly, although not specific, is an important sign of torsion in the setting of a wandering spleen. A markedly hypodense spleen due to infarction may be misdiagnosed as a mesenteric cyst, a dilated fluid-filled bowel loop, or an abscess. Torsion of a wandering spleen may be associated with intestinal obstruction (due to compression of other abdominal organs), abscess formation, necrosis of the pancreatic tail, and recurrent pancreatitis.
Magnetic resonance imaging (MRI) has gained popularity in diagnosis of wandering spleen. It can locate the ectopic spleen and identify the viability of the splenic parenchyma as well as depict splenic vessel anatomy.
Angiography
Although it is not usually required for diagnosis, celiac arteriography may show torsion of wandering spleen by demonstrating a tapered and abruptly twisted distal splenic artery. The venous phase may suggest splenic vein obstruction and may show collateral circulation and varices. MR angiography can be particularly useful in demonstrating the location and length of the splenic artery before surgery.
Diagnostic Approach and Therapy
When wandering spleen is suspected, radiologic imaging should locate the ectopic spleen and evaluate the anatomy and patency of the splenic vessels. Evaluation of the viability of the splenic parenchyma, including any areas of infarction, is important as it guides therapy.
The definitive therapy for a wandering spleen is surgery. Splenopexy with splenic salvage is the preferred treatment in cases in which the spleen is viable after detorsion and the splenic vein is not thrombosed. Splenectomy is the option in cases of splenic infarction.
Accessory Spleen
Accessory spleen is a congenital anomaly caused by failure of embryonic splenic buds to unite within the dorsal mesogastrium and extreme lobulation of the spleen with pinching off of splenic tissue. Its reported incidence ranges from 10% to 30% in autopsy series. Accessory spleen is identified in 11% of patients undergoing contrast-enhanced CT and 45% to 65% of patients after splenectomy.
Accessory splenic tissue measures between 1 and 3 cm in diameter but can be as large as the spleen. It is usually located about the splenic hilum or ligaments. The tail of the pancreas is the second most common site for an accessory spleen, accounting for 16% of the cases. Accessory spleens can be found in the wall of the stomach, the large bowel, the omentum, the mesentery, and rarely even the scrotum.
The morphologic appearance is identical to that of normal spleen, and the accessory tissue is usually supplied by a branch of the splenic artery and drains into splenic veins. Of the patients with an accessory spleen, 88% were solitary, 8% had two, and 3% had three.
Clinical Findings
Accessory spleen is an incidental finding of no clinical significance in most patients. However, it can become symptomatic because of torsion or spontaneous rupture. Accessory spleen is important to identify as it may mimic lymph nodes or tumors, especially in the tail of the pancreas.
Identification of all accessory splenic tissue is important in the setting of idiopathic thrombocytopenic purpura, in which all functional splenic tissue should be removed for the treatment of the hematologic disorder.
Radiologic Findings
Nuclear scintigraphy, sonography, CT, and MRI can identify and characterize accessory splenic tissue. The normal accessory spleen has the same echotexture and enhancement as normal spleen. When the tissue is recognized as an accessory spleen, no further work-up is needed. Diagnostic studies are indicated if this tissue simulates a neoplasm or if therapeutic splenectomy is planned in patients with hematologic disorders.
On gray-scale ultrasound, accessory spleen is identified as a round or oval mass about the splenic hilum that is identical to spleen in echotexture. On color or power Doppler ultrasound, a vascular hilum entering the lesion is a diagnostic feature of accessory spleen. Contrast-enhanced ultrasound has been shown to demonstrate accessory spleen with an enhancement pattern paralleling that of the adjacent spleen.
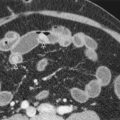
On multiphase contrast-enhanced CT, an accessory spleen is usually identified as a well-circumscribed mass about the splenic hilum with an inhomogeneous striped arterial enhancement pattern identical to the spleen ( Fig. 104-5 ). Intrapancreatic accessory spleen can mimic pancreatic neoplasms, such as hypervascular islet cell tumors. The attenuation of the spleen is typically higher than that of the pancreas on the arterial, pancreatic, and portal venous phases, and similarly, intrapancreatic accessory spleen tends to be higher in attenuation than pancreas on all phases of imaging. Conversely, hypervascular pancreatic tumors, including islet cell tumor, are hyperattenuating on the arterial phase and isoattenuating or lower attenuating to the adjacent pancreas on the venous phase.

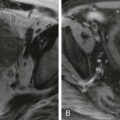
On MRI, accessory spleen is isointense to mildly hypointense to spleen on T1 imaging and isointense to spleen on T2 imaging. Its enhancement pattern is identical to that of spleen on arterial, portal, and late phases of contrast enhancement ( Fig. 104-6 ). Accessory spleen is isointense to spleen on diffusion-weighted imaging. The identical MRI features of accessory spleen to spleen allow the discrimination from pancreatic neoplasms.

99m Tc-labeled heat-damaged red blood cell ( 99m Tc-HDRBC) scintigraphy is a highly specific method for detection of splenic tissue as up to 90% of the injected HDRBCs are trapped by splenic tissue. 99m Tc-HDRBC scintigraphy allows selective splenic visualization with an excellent spleen-to-liver ratio; splenic visualization may be impaired when minimal functioning splenic tissue is present, as happens in cases of accessory spleens. In addition, 99m Tc-HDRBC planar imaging scintigraphy and even single photon emission computed tomography (SPECT) offer inferior spatial resolution compared with other cross-sectional imaging modalities. 99m Tc-HDRBC SPECT is frequently used in conjunction with other cross-sectional imaging modalities to diagnose and to precisely localize an accessory spleen (see Fig. 104-6 ).
Splenic-Gonadal Fusion
This rare congenital anomaly is characterized by an abnormal connection between the gonad and the spleen or by ectopic splenic tissue. The left gonad is virtually always involved; only one case of right-sided involvement has been reported. Before the sixth week of gestation, the splenic anlage developing in the left dorsal mesogastrium is close to the left urogenital fold that contains gonadal mesoderm during rotation of the embryonic gut. The accepted theory is that splenic-gonadal fusion is the abnormal attachment that occurs during the fifth to eighth week of gestation when both organs are close. Splenic-gonadal fusion occurs with a male-to-female ratio of 16 : 1 and can interfere with the normal descent of the left testis or can cause failure of closure of the processus vaginalis; it is frequently associated with an ipsilateral inguinal hernia and cryptorchidism.
The continuous form, in which the spleen remains attached to the gonad by a fibrous or splenic cord that traverses the peritoneal cavity, is the most common type, occurring in about 55% of the published cases. Continuous splenic-gonadal fusion is associated with limb defect syndrome and other congenital malformations, including cardiac malformations, thoracic malformations, cleft palate, micrognathia, anal anomalies, spina bifida, craniosynostosis, diaphragmatic hernia, and pulmonary anomalies. The discontinuous form, representing 45% of the cases, is not associated with other congenital anomalies.
The diagnosis of splenic-gonadal fusion should be suspected if there is chronic swelling or mass in the left gonad, especially if there is an associated undescended testis. The gonadal mass, if palpable, is firm and rubbery and may be poorly delineated from the testis. The first study is ultrasound, which can show a well-defined scrotal mass that is isoechoic to spleen. Malignant disease associated with splenic-gonadal fusion is exceedingly rare and occurs in the undescended testis.
99m Tc-sulfur colloid scintigraphy is useful to confirm ectopic splenic tissue and shows uptake in the inguinal or scrotal area.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree