CHAPTER 46 Anomalous Pulmonary Venous Connections and Drainage
Controversy exists about the origin of the primordial pulmonary vein.1 Regardless, it is generally accepted that a common pulmonary vein forms in the dorsal mesocardium and is progressively incorporated into the posterior wall of the left atrium. As the atrium expands and the common vein is absorbed, the four major branches (two left and two right) achieve their discrete insertions.
PARTIAL ANOMALOUS PULMONARY VENOUS CONNECTION
Prevalence and Epidemiology
The incidence of PAPVC at autopsy has been reported to range between 0.6% and 0.7%.2,3 The clinical frequency is less, indicating that many cases remain asymptomatic. Ethnic and gender predilections are unknown, probably because of the relative infrequency of this process.
Etiology and Pathophysiology
The anatomic manifestations of PAPVC are varied. Anomalous connections can occur both above and below the diaphragm, generally to an ipsilateral systemic vein. The right-sided pulmonary veins most often drain into the embryologic derivatives of the right cardinal vein, usually the inferior vena cava (IVC) or superior vena cava (SVC). Anomalous left-sided veins typically empty into derivatives of the left cardinal vein, most often the left innominate vein or the coronary sinus. Anomalous veins may also connect through remnants of the primitive splanchnic plexus to contralateral systemic vessels, although this is less common. Connection of a pulmonary vein (usually the right upper lobe branch) to the posterior SVC at the junction of the right atrium due to a defect of the common wall between the SVC and the right upper lobe pulmonary vein represents a unique process known as a sinus venosus defect (see following section). Drainage of the right pulmonary veins to the IVC may be associated with systemic arterial supply and hypoplasia of the ipsilateral lung with secondary dextroposition of the heart. This condition, variably referred to as congenital venolobar syndrome or scimitar syndrome (Fig. 46-1), is often placed within the continuum of bronchopulmonary dysplasias.4,5 A horseshoe lung refers to the fusion of the lower lobes across the midline without an intervening fissure. This anomaly is highly associated with scimitar syndrome, with up to 80% of patients with horseshoe lung also affected with PAPVC in some series.5
Manifestations of Disease
Imaging Indications and Algorithm
The suggestion of anomalous venous return on chest radiography or echocardiography warrants further evaluation with cardiac CT angiography (CTA) or MRI. The choice of which cross-sectional modality to employ varies with institution. Patient factors (such as contraindications to MRI or contrast media) must also be considered. In addition, because of the increased frequency of PAPVC reported in patients with Turner syndrome, routine screening in this population may be warranted.6
Imaging Techniques and Findings
Radiography
Signs of right ventricular overload, including a lateralized or upturned cardiac apex on the frontal radiograph and filling in of the retrosternal clear space on the lateral view, may be evident. Increased pulmonary blood flow is often present; however, it is nonspecific. The abnormal vein itself may be visualized, as in anomalous pulmonary venous drainage of the right lung to the IVC, when the so-called scimitar vein (named for the crescent shape of the vessel likened to a Turko-Mongol saber; Fig. 46-2) can be visualized. As described previously, patients with scimitar syndrome display hypoplasia of the right lung and often some degree of cardiac dextroposition. Aberrant drainage into the SVC or azygos vein may result in dilation of these structures that is radiographically apparent. Anomalous left pulmonary veins emptying into the left innominate vein can create a bulbous appearance of the superior mediastinum (Fig. 46-3).
Ultrasonography
Echocardiography is often the first imaging modality employed to evaluate a child with suspected structural heart disease. Defining the pulmonary venous connections is an integral step in every echocardiographic examination. The pulmonary venous connections are often best demonstrated through a subxiphoid approach in infants. Suprasternal, parasternal, apical, and subcostal windows are often more revealing in older children. When not all of the pulmonary venous connections can be accounted for, a more detailed search for systemic connections is required. The presence of dilated systemic veins can be a clue to an unsuspected anomalous venous connection. Right ventricular volume overload resulting from PAPVC is well evaluated with echocardiography. Because of limitations in field of view of the pulmonary veins and left atrium from a transthoracic technique, transesophageal scanning may be needed for more detailed anatomic study of the pulmonary vein insertion.7
Computed Tomography
CTA timed to maximize left atrial and pulmonary venous opacification provides high spatial resolution images of the course and connections of anomalous pulmonary veins.8 The isotropic acquisition of current multidetector CT scanners allows multiplanar reformation, maximum intensity projection, and volume rendered reconstruction. These techniques provide a degree of anatomic visualization previously available only with angiography. In addition, surrounding noncardiac structures, such as the lung parenchyma, are well demonstrated. This can aid in the characterization of an associated hypoplastic or horseshoe lung (see Fig. 46-1).
Magnetic Resonance
Cardiac MRI is another useful tool for the evaluation of PAPVC. MRI has been shown to be accurate in the evaluation of pulmonary vein anomalies and is often considered the method of choice for preoperative characterization of PAPVC (see Fig. 46-3).9–11 Black blood images, like CTA, provide high spatial resolution for the evaluation of anatomic connections. Phase contrast cine images allow accurate quantification of pulmonary and systemic blood flow for shunt fraction calculation. Whereas the evaluation of lung parenchyma is less optimal than with CT, the lack of ionizing radiation and the functional information about the quantification of right ventricular volume overload and shunt fraction make MR superior for guiding clinical management.
Synopsis of Treatment Options
Surgical/Interventional
Indications for surgical treatment are controversial. Generally speaking, all patients who are symptomatic and do not have a contraindication should be treated surgically. Specifically, the current consensus holds that if the Qp:Qs (shunt fraction) is greater than 1.5 : 1, surgical closure is performed. PAPVC with shunt ratios below 1.5 : 1 are usually well tolerated and can be clinically observed. The particular repair will vary according to the site of anomalous drainage and the coexistence of any other form of heart disease. Anomalous left pulmonary veins may be reanastomosed to the left atrial appendage. Right-sided anomalous veins are often anastomosed to the right atrium and connected to the left atrium with a patch or baffle through a preexisting or surgically created ASD. In general, surgical repair of PAPVC is associated with very good outcomes.12 Patients with scimitar syndrome, however, often do poorly and suffer from high degrees of postoperative pulmonary venous stenosis related to baffle obstruction (Fig. 46-4).12
Reporting: Information for the Referring Physician
KEY POINTS
 PAPVC results from failure of at least one of the pulmonary veins to become incorporated into the left atrium.
PAPVC results from failure of at least one of the pulmonary veins to become incorporated into the left atrium. The anomalous pulmonary vein can connect to a variety of systemic veins, including the IVC, SVC, innominate and azygos veins, and coronary sinus.
The anomalous pulmonary vein can connect to a variety of systemic veins, including the IVC, SVC, innominate and azygos veins, and coronary sinus. Patients with PAPVC can present with dyspnea on exertion or murmur related to right-sided heart enlargement and volume overload.
Patients with PAPVC can present with dyspnea on exertion or murmur related to right-sided heart enlargement and volume overload. CT or MR angiography is the noninvasive imaging method of choice for delineating anomalous pulmonary venous connections.
CT or MR angiography is the noninvasive imaging method of choice for delineating anomalous pulmonary venous connections.SINUS VENOSUS DEFECT
Prevalence and Epidemiology
The SVD has been estimated to account for up to 10% of ASDs.13 The exact prevalence is difficult to measure because of the often subclinical nature of the condition and the difficulty in detection with standard first-line cardiac imaging techniques (plain radiography and echocardiography). Most studies have found a female-to-male preponderance of roughly 2 : 1. No racial or ethnic predilection has been established.
Etiology and Pathophysiology
The lack of an intact intervening wall between a pulmonary vein and the SVC or right atrium results in “unroofing” of the pulmonary vein, thereby creating anomalous pulmonary venous drainage to the right atrium.14 An interatrial connection posterior and superior to the fossa ovalis is commonly present. This does not represent an ASD, but rather it is a connection between the atria formed by the unroofed insertion of the pulmonary vein. Because of higher left-sided pressures, flow of blood can pass retrograde from the left atrium through the orifice of the pulmonary vein and then enter the SVC through the defect and continue to the right atrium, resulting in a left-to-right shunt. Additional anomalous connecting pulmonary veins to the SVC superior to the defect as well as other systemic veins may be present.
Manifestations of Disease
Clinical Presentation
The often large interatrial communication of an SVD results in a significant left-to-right shunt with increased pulmonary blood flow; some patients can be relatively asymptomatic while others may present with congestive heart failure.13,14 Symptoms include dyspnea on exertion, palpitations, and angina.13,15 As with conventional ASDs, SVD may be the cause of an otherwise unexplained stroke due to paradoxic embolization.15
Imaging Techniques and Findings
Radiography
Plain film findings in SVD are nonspecific but include cardiomegaly with right-sided heart enlargement and increased pulmonary blood flow (Fig. 46-5).

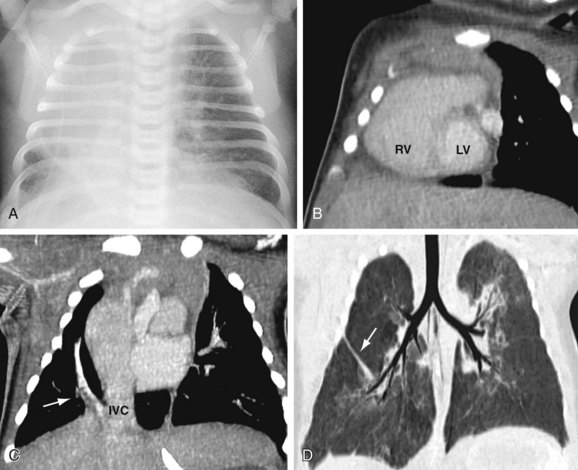
 FIGURE 46-1
FIGURE 46-1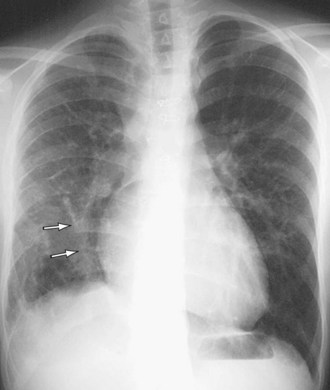
 FIGURE 46-2
FIGURE 46-2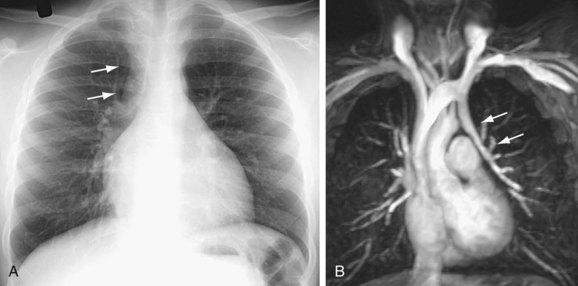
 FIGURE 46-3
FIGURE 46-3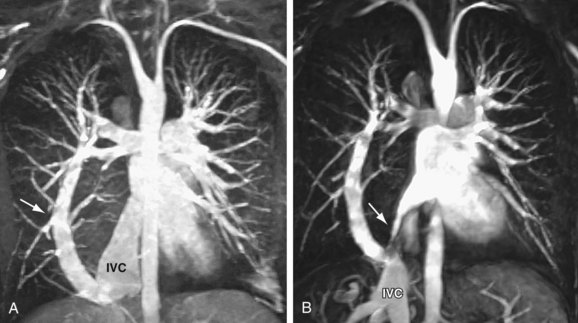
 FIGURE 46-4
FIGURE 46-4
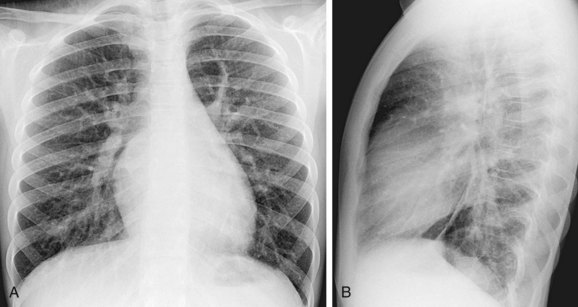
 FIGURE 46-5
FIGURE 46-5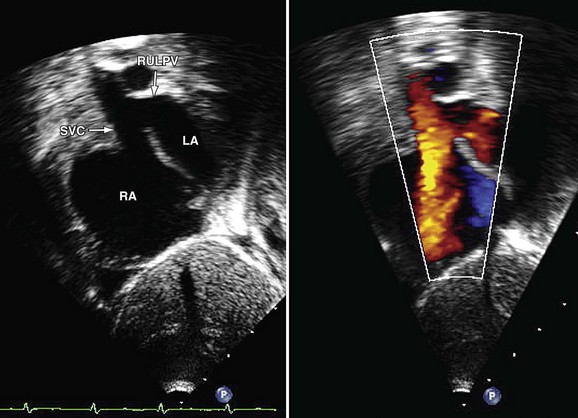
 FIGURE 46-6
FIGURE 46-6

