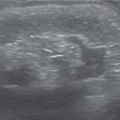
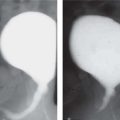
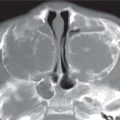
Duplex kidney
Fig. 3.9
Fig. 3.10 |
US: Large kidney with subtle duplication of renal collecting systems and echogenic central sinus complexes. May show ureterocele in bladder.
EU: Two renal pelves and ureters. The ureters may fuse distally. |
Frequency is approximately 1%. May be complete (major) duplex or partial (minor) duplex. Complete duplex systems may show upper pole obstruction and lower pole reflux. Upper pole tends to insert ectopically in the bladder and be associated with an ureterocele (Weigert-Meyer rule). |
Compensatory hypertrophy (solitary kidney) |
Large but normal kidney with abnormal or absent kidney on the other side. |
Secondary to decreased renal function in the opposing kidney. |
Nephromegaly |
Large, normal appearance. No duplication of collecting system. |
Associated with hemihypertrophy, Beckwith Wiedemann syndrome, Perlman syndrome. |
Crossed renal ectopia
Fig. 3.11a, b |
(see agenesis, dysplasia, and ectopia in Table 3.10 ) |
|
Renal contusion/laceration
Fig. 3.12a, b |
CECT: Wedge-shaped areas of decreased attenuation. Will show extent of hemorrhage, renal perfusion, and acute ongoing hemorrhage. Delayed CT will show damage to pelvis/ureters and continuity of ureters. |
Secondary to blunt abdominal trauma. CT is the modality of choice and allows assessment of other areas. |
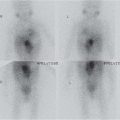
Acute pyelonephritis
Fig. 3.13 |
US: May be normal. Renal swelling, loss of corticomedullary differentiation, focal areas of abnormal echogenicity. Thickening of walls of pelvis/ureter and renal sinus hypoechogenicity.
Doppler: wedge-shaped areas of hypoperfusion.
DMSA: focal/diffuse decrease in tracer uptake. |
Most commonly upper pole.
Imaging has limited role but may show structural abnormality that predisposes to infection or complications such as abscess. |
Acute obstructive nephropathy |
US: variable dilatation of collecting system.
CT: may show calculus.
CECT/EU: persistent nephrogram and swollen parenchyma.
MRI: persistent nephrogram and delayed renal transit. MAG3: delayed renal transit time. |
Causes: calculus, acute obstruction of partially obstructed system. |
Nephroblastomatosis
Fig. 3.14 |
US: enlarged kidney with hypoechoic nodules and loss of corticomedullary differentiation.
CECT/MRI: Low attenuation nodules with poor enhancement. Predominantly subcapsular. Can be diffuse. |
Usually bilateral.
Neonate to 2 y. Increased risk of Wilms tumor, especially with Beckwith-Wiedemann syndrome. Requires follow-up but can spontaneously regress. |
Mesoblastic nephroma
Fig. 3.15a, b |
US: predominantly solid hypoechoic/mixed echogenicity lesion.
Doppler: may show anechoic vascular ring around tumor.
CT: enhances postcontrast but less than normal renal parenchyma. |
Cysts, hemorrhage, and necrosis rarely found.
Most common neonatal solid tumor. Ninety percent of patients are aged < 1 y.
Although benign, can rarely metastasize to lungs, brain, and bone. |
Wilms tumor |
US/CT/MRI: heterogeneous mass. Inferior vena cava and renal vein may show thrombus. Does not encase vessels. Nodal metastases. Low signal on T1-weighted and high on T2-weighted MRI. MRI most sensitive for caval patency. |
Eighty percent occur before 5 y of age.
Four to thirteen percent are bilateral; 1% are familial. Five to ten percent have calcifications.
Associated with cryptorchidism, hemihypertrophy, hypospadias, sporadic aniridia. |
Multilocular cystic renal tumor |
US/CT/MRI: Well-demarcated cystic intrarenal mass. Septae enhanced. Cysts may show evidence of hemorrhage or protein on MRI. |
Two age peaks: 3 mo to 4 y (boys > girls). Adults (mainly women).
Benign. |
Autosomal dominant polycystic kidney disease (ADPKD)
Fig. 3.16a, b |
See Table 3.6 |
May be unilateral initially in young patients. |
Renal transplant rejection |
US: Increased echogenicity. Loss of corticomedullary differentiation.
Doppler: decreased blood flow and increased resistive indices. |
Acute tubular necrosis has a similar appearance. |
Medullary sponge kidney
Fig. 3.17 |
(see bilateral medullary sponge kidneys, Table 3.6 ) |
|
Xanthogranulomatous pyelonephritis
Fig. 3.18a, b |
US/CT: Calcified nodule in otherwise normal kidney seen in localized form. Diffuse form shows large lesion replacing entire kidney with loss of corticomedullary differentiation, cystic/necrotic areas, calcification/stones around renal pelvis. |
Severe atypical, chronic renal parenchymal infection. Multiple causes: 70% stones. Diff cult to differentiate from tumor. |
Acute renal vein thrombosis
Fig. 3.19 |
US: Enlarged, hypoechoic kidney with hyperechoic streaks. Loss of corticomedullary differentiation. Thrombus not always visible.
Doppler: Lack of flow in renal vein. Absent/reversed end-diastolic flow in intrarenal arteries.
MRI: most sensitive if US equivocal. |
May be bilateral. On the left, may be associated with adrenal hemorrhage.
Causes: asphyxia, shock, dehydration, infant of diabetic mother. |