1 Anterior Cranial Fossa, Nasal Cavity, and Paranasal Sinuses The anterior skull base, nasal cavity, and paranasal sinuses can be affected by a diverse group of neoplastic and nonneoplastic pathology that may transgress the boundaries between these or other adjacent spaces. Understanding the close relationship, patterns of disease spread, and potential complications is essential for optimal diagnostic evaluation of this area. In the case of malignant disease, accurate identification of tumor extent is essential for appropriate staging and preoperative planning and to ensure an optimal resection margin and the best possible outcome for the patient. In this chapter, we will provide an overview of the imaging characteristics of the large array of common and uncommon pathology affecting these regions. However, beyond the morphologic imaging characteristics, we will emphasize clinically important features or patterns of spread, supplemented by commonly used current treatment paradigms. In doing so, it is our hope not to be limited by imaging findings alone but rather focus and emphasize those findings that have the greatest clinical relevance and impact on patient care. In order to help better understand the anatomic pathways of disease spread and anatomic variants that can predispose to complications and are of importance for surgical planning, this chapter will begin with a brief overview of embryologic development, followed by a clinically oriented review of imaging anatomy. The formation of the structures constituting the face, paranasal sinuses, and skull base is achieved through an elaborate, highly orchestrated series of cell migration, proliferation, and differentiation controlled by complex cascades of cell signaling systems. A detailed discussion of the embryological development of the anterior skull base and paranasal sinuses is beyond the scope of this chapter and covered in detail elsewhere.1,2 The purpose of this section is to provide a brief and broad overview of the development of these structures to highlight some key milestones and structures that may help the reader understand important anatomic landmarks, functional units, and basis for variations that may be encountered in clinical practice. The cartilaginous neurocranium or chondrocranium forms the base of the developing skull through fusion of adjacent cartilages. The skull base is formed mainly by endochondral ossification, whereas the calvarial vault is mainly formed by membranous ossification.2,3 Numerous ossification centers are involved in the development of the skull base, but the major ossification centers present at birth are the basioccipital center, the basisphenoidal center, and the presphenoidal center giving rise to the basiocciput, basisphenoid (postsphenoid), and presphenoid, respectively. The orbitosphenoid cartilage, alisphenoid cartilage center, and condensed mesenchyme (intramembranous bone) give rise to the more lateral parts of the sphenoid/sphenoid wings.4 At birth, there is very limited ossification of the anterior skull base.5 Ossification of the skull base occurs in an orderly fashion in the first 2 years of life from posterior to anterior and lateral to medial, and by 24 months of age, the anterior skull base is nearly completely ossified. However, small gaps may persist in the nasal roof until 3 years of age and the foramen cecum may ossify as late as 5 years of age.3 Nasal cavity and subsequent paranasal sinus development begins at the frontonasal prominence with the formation of bilateral oval thickenings of the surface ectoderm, referred to as nasal placodes, that are present by the end of the fourth week.1,2 Subsequently, these recede into flat depressions called nasal pits (the primordia of anterior nares or future nostrils). From the fifth week of gestation, the nasal pits deepen toward the oral cavity, eventually resulting in an open communication between the oral and nasal cavities. An elaborate cascade of growth and fusion then leads to the downward growth of the nasal septum, formation of the definitive secondary palate, and other structures constituting the nasal cavity. Ectodermal epithelium at the roof of the nasal cavity differentiates into the olfactory epithelium, with the development of olfactory receptor cells having axons that lead into the developing olfactory bulbs. All paranasal sinuses begin as evaginations or diverticula located along the walls of the nasal cavities that evolve into air-filled cavities in the adjacent bones.6 As such, they are lined by a pseudostratified columnar-ciliated epithelium similar to that lining the nasal cavity. In adults, the diverticular origins persist as the sinus orifices. The maxillary sinus is the first paranasal sinus to form during fetal life. At birth, the maxillary sinuses are rudimentary and grow slowly until puberty. Full development of the maxillary sinuses occurs after eruption of permanent maxillary dentition at approximately 20 years of age.7 The ethmoid air cells are the first sinuses to fully develop. At birth, the ethmoid air cells are fully developed in number but not in size. Thereafter, there is progressive development and growth of the ethmoid air cells, and by approximately 12 years of age, the ethmoid air cells have reached their adult size.6 The frontal sinus first begins with the appearance of the frontal recess in the lateral nasal cavity wall.6 A series of pits or furrows then develop in the frontal recess that represent rudimentary ethmoid air cells, each of which has the potential to form the frontal sinus. Occasionally, the frontal sinus may also develop from an ethmoid air cell that has migrated from the ethmoid infundibulum rather than the frontal recess. The main development of the frontal sinus occurs in the postnatal period, with the sinus slowly enlarging to reach maximal adult size near the end of the second decade of life.6 At birth, the sphenoid sinus is devoid of air, containing red marrow. Conversion of red to fatty marrow occurs between 7 and 24 months of age and is believed to first start in the presphenoid.8,9 Marrow conversion then extends more posteriorly into the basisphenoid. The sphenoid sinus starts its major growth in the third to fifth year of life, typically reaching adult configuration by the age of 12 years.6 The process of pneumatization is typically preceded by fatty marrow conversion. In rare cases, premature arrest of sinus development is proposed as a potential etiology for alterations in the appearance of the skull base that can mimic pathology, referred to as arrested pneumatization of the skull base.10 These are typically located in the basisphenoid or adjacent skull base. They appear nonexpansile with sclerotic borders, internal fat, and internal curvilinear calcifications and should be recognized as a developmental variant and not misinterpreted as a pathologic process ( The anterior skull base can be broadly described as constituting the floor of the anterior cranial fossa and the roof of the nose, ethmoid air cells, and orbits.3,11 Posteriorly, the anterior skull base is formed by the lesser wings and anterior body of the sphenoid bone, including the planum sphenoidale, tuberculum sella, and anterior clinoid processes. The bones forming the anterior skull base are the ethmoid (cribriform plate) centrally and the horizontal portion (orbital plate) of the frontal bone more laterally, the latter forming the greater part of the anterior cranial fossa ( Fig. 1.1 Arrested pneumatization of the sphenoid sinus. Coronal reformatted images from a high-resolution contrast-enhanced CT displayed in bone (a,b) and soft-tissue windows (c) demonstrate a nonexpansile, nonaggressive lesion in the basisphenoid with sclerotic borders and internal curvilinear densities/calcifications (arrow). The lesion is broader on the left side. A fatty component is clearly seen medially in the lesion on CT (c) and high-signal fatty content of the lesion is even better seen on the coronal T1-weighted MR images (d,e), with suppression of fat signal resulting in low signal on the axial fat-suppressed T2-weighted image (f). Fig. 1.3 Anatomy of the skull base, extracranial view. Illustration of the extracranial aspect of the skull base, with the orbital roofs, ethmoid air cells, and sphenoid sinus exposed. The anterior skull base can be broadly described as constituting the floor of the anterior cranial fossa, roof of the nose, ethmoid air cells, and orbits anteriorly and the lesser wings and anterior body of the sphenoid bone posteriorly. Cribriform plate/ethmoid bone (purple), frontal bones (tan), occipital bone (green), parietal bones (pink), sphenoid bone (red), temporal bones (grey). Other bony landmarks of the anterior skull base include the frontal crest and crista galli. The frontal crest is a midline ridge between the frontal bones anteriorly and serves as a site of attachment for the falx cerebri ( The foramina of the anterior skull base include those in the cribriform plate, the foramen cecum, and the anterior and posterior ethmoidal foramina ( The anterior ethmoidal neurovascular bundles (artery, vein, and nerve) enter the cranial cavity at the junction of the cribriform plate and the orbital plates of the frontal bone anteriorly on each side. Similarly, the posterior ethmoidal canals and attendant neurovascular contents are transmitted through the skull base at the junction of the posterolateral corners of the cribriform plate and the sphenoid. The anatomic variations in the trajectory of these structures, well seen on high-resolution sinus computed tomography (CT), can be important for surgical planning during endoscopic surgery and will be discussed in more detail in the section on paranasal sinuses. The anterior skull base has important relations to critical adjacent structures and spaces that need to be taken into account when evaluating different pathology as well as for treatment planning. Superiorly, these are the structures of the anterior cranial fossa, including the frontal lobes as well as the olfactory bulb and tract (olfactory nerve or cranial nerve 1). Inferiorly, these are the nasal cavity and ethmoid air cells medially and the orbits laterally. The applied anatomy of the nasal cavity and paranasal sinuses will be discussed in the next section. The nasal cavity is a triangular area divided on each side by the nasal septum ( The olfactory fossa is formed by the cribriform plate, separated at the midline by the crista galli. The cribriform plate has a medial lamella inferiorly and medially and a lateral lamella that is vertical or slanted ( Fig. 1.5 Coronal reformat from a high-resolution sinus CT demonstrates basic anatomy of the cribriform plate and triangular-shaped nasal cavity (solid white lines). The cribriform plate (red solid and dotted lines) forms the olfactory fossa (asterisk) and the roof of the horizontal central part of the nasal cavity. It consists of a medial lamella (red solid line) and a lateral lamella (red dotted line) that are separated by the vertical lamella of the middle turbinate (arrowheads). Laterally, the cribriform plate becomes contiguous with the roof of the ethmoid labyrinth, the fovea ethmoidalis (FE), formed by the orbital plate of frontal bone. The nasal cavity is divided into two compartments by the nasal septum. The nasal septum consists of a longer, posterior bony component and a shorter anterior cartilaginous component ( Laterally, the nasal cavity consists of the lateral nasal wall and the attached turbinates, whereas the floor of the nasal cavity is formed by the hard and soft palate.3,4 The lateral nasal cavity is formed by multiple bones, including the maxilla, the ethmoid bone, the perpendicular plate of the palatine bone, the lacrimal bone, the medial pterygoid plate, and the inferior nasal concha. The inferior nasal concha is a separate bone, whereas the superior and middle turbinates are extensions of the ethmoid bone, as is the uncinate process. The bony labyrinth of the ethmoid is at the superolateral boundary of the nasal cavity. There are three nasal conchae arising from the lateral or superior nasal cavity margins.3,4,9,23 Together, the bony nasal conchae and the vascular mucosa and submucosa lining them are referred to as turbinates. The three main turbinates are the superior, middle, and inferior turbinates, with the inferior turbinate being the largest among them ( The superior concha or turbinate is a small, curved osseous lamina posterosuperior to the middle turbinate ( Fig. 1.6 Keros classification and other important variants of the fovea ethmoidalis—examples on sinus CT. Coronal reformats from sinus CT scans of three different patients demonstrate variations in the depth of the olfactory fossa, as defined by the height of the lateral lamina of the cribriform plate (red dotted line). Based on the Keros classification, there are three types: (a) 2 mm (type 1: 1–3 mm); (b) 4 mm (type 2: 4–7 mm); and (c) 10 mm (type 3: 8–16 mm). Greater depth corresponds to a greater risk of iatrogenic injury. In addition to variations in the olfactory fossa depth, there is asymmetry of the ethmoid roof as well as a flat sloping shape of ethmoid roof on the right (c; arrow), with an increase in the angle between the fovea ethmoidalis and the lateral lamina of the cribriform plate approaching 180 degrees. Both of these variants can be associated with an increased risk of iatrogenic injury and complications during endoscopic surgery. Fig. 1.8 Nasal turbinates. (a) Coronal and (b) sagittal reformats from a sinus CT demonstrating the superior (ST), middle (MT), and inferior (IT) nasal turbinates and their respective meati (SM, superior meatus; MM, middle meatus; IM, inferior meatus). On the sagittal image (b), the superior turbinate is not seen because it is medial to the plane of the section. The middle turbinates have a particularly complex anatomy and understanding this anatomy is key for understanding the functional units and drainage pathways in the paranasal sinuses.27,28 The attachment of the middle turbinates to adjacent bones has components in all three planes ( One of the most common anatomic variations of the middle turbinate is pneumatization of that turbinate ( Fig. 1.9 Attachments of the middle turbinate. (a–c) Coronal reformats (anterior to posterior) and (d,e) sagittal reformats from a sinus CT demonstrate the complex anatomy of the middle turbinate with attachments to adjacent bones that has components in all three planes (red dotted lines). There is variation in terminology used, some referring to all parts as the basal lamella of the middle turbinate and others referring to the anterior part as the vertical lamella and the middle and posterior parts as the basal lamella. Regardless, there is an anterior vertical portion in a near sagittal plane that attaches to the cribriform plate between the medial and lateral lamella of the cribriform plate (a,b). More posteriorly, the middle portion lies in a nearly coronal plane and attaches to the lamina papyracea and the most posterior portion lies in a nearly horizontal plane and attaches to the lamina papyracea, medial maxillary sinus wall, or both (b–e). It should also be noted that the basal lamella of the middle turbinate is the landmark used for separating anterior (white asterisks) from posterior (blue asterisks) ethmoid air cells (d,e). FS, frontal sinus; SS, sphenoid sinus. The superior meatus is a narrow passage between the superior and middle turbinates ( Fig. 1.11 Sphenoethmoidal recess and related drainage pathways. The sphenoethmoidal recess (red dotted line), the recess between the superior concha (white arrow) and the body of the sphenoid, receives drainage from the sphenoid sinus as well as from the posterior ethmoidal air cells (via the superior meatus). The red arrow marks the sphenoid ostium. IT, inferior turbinate; MT, middle turbinate; SM, superior meatus; SS, sphenoid sinus. The middle meatus is wider than the superior meatus ( Much of the nasal cavity and paranasal sinuses are lined by pseudostratified ciliated (respiratory) epithelium containing goblet cells. The olfactory cleft and adjacent regions are lined by olfactory epithelium, and there is stratified squamous epithelium anteroinferiorly in continuity with the nares. The mucosa is adherent to the adjacent periosteum or perichondrium, sometimes referred to as the mucoperiosteum. Seromucous glands of the nasal mucosa secrete a mucous film which is moved posteriorly into the nasopharynx by ciliary action. The lamina propria also contains cavernous venous vascular tissue, thickest over the conchae. Paranasal sinus mucosa is thinner, less vascular, contains fewer goblet cells, and is more loosely attached to underlying bones. Fig. 1.12 Ostiomeatal unit (OMU). Coronal reformat from a sinus CT demonstrating the main components of the OMU as seen in the coronal plane. The OMU refers to a functional unit of structures draining the frontal, anterior ethmoid, and maxillary sinuses. It includes the middle meatus, the ethmoid bulla (EB), the uncinate process (blue arrows), hiatus semilunaris (red arrow), the infundibulum (dotted line), and the superomedial maxillary sinus/maxillary sinus ostium (white arrow). The arterial supply of the nasal cavity is derived from branches of the ophthalmic, maxillary, and facial arteries which ramify to form anastomotic plexuses within and deep to the nasal mucosa. Venous drainage of the cavernous tissue involves the sphenopalatine vein, veins accompanying the anterior ethmoidal arteries which lead to the cavernous sinuses, and, in cases of a patent foramen caecum, a nasal vein communicating with the superior sagittal sinus as discussed earlier. Lymphatic drainage from the anterior nasal cavity joins that of the skin, and the primary nodal groups draining this area are those in the submandibular region (level IB lymph nodes). The primary nodal drainage areas for the posterior two-thirds of the nasal cavity and paranasal sinuses are the retropharyngeal lymph nodes and the level II and III lymph nodes.32 The posterior aspect of the nasal floor may also drain into parotid lymph nodes.11 Nasal cavity general sensory innervation is mediated by branches of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerves, including anterior ethmoidal, infraorbital, anterior superior alveolar, greater palatine, nasopalatine, and Vidian nerves. Autonomic innervation occurs by sympathetic vasomotor fibers following the distribution of blood vessels. Parasympathetic secretomotor functions are mediated by branches of the maxillary nerves. This includes contributions from the Vidian nerve, which provides parasympathetic innervation to the nose and lacrimal gland. This is clinically important and if this nerve requires sacrifice as part of the patient’s surgery, it can result in an ipsilateral dry eye. As such, when at risk, this will have to be discussed with the patient during the consenting procedure. The olfactory nerves form a plexiform network in the subepithelial lamina propria of the superior septal and turbinate mucosa. This network coalesces into as many as 20 separate bundles which traverse the cribriform plate in lateral and medial groups to form the olfactory bulbs. At this level, the dura is continuous with the nasal periosteum. The paired frontal sinuses are located between the cortical tables of the frontal bone. Each frontal sinus usually underlies a triangular portion of the face, though rarely in a symmetrical configuration. The frontal sinuses on either side are separated from one another by the intersinus septum, although frequently the septum is not exactly midline and the larger sinus crosses the midsagittal plane to the contralateral side. The majority of time, the intersinus septum is complete, but occasionally there may be focal defects allowing intercommunication between the sinuses. The septum may also have an intersinus septal cell. These are believed to arise from the frontal sinuses themselves, rather than migration of anterior ethmoid air cells. The sinuses may, in addition, be divided into numerous interconnected recesses by incomplete bony septa or intrasinus septa. The primary ostium of the frontal sinus is located medially and drains through the frontal recess into the middle meatus, discussed in detail in the section on sinus drainage and frontoethmoidal recess later. The pneumatization of frontal sinuses is variable. These sinuses typically extend a short distance above the medial aspect of the eyebrow and, posteromedially, they may reach into the orbital roof as far as the lesser wing of the sphenoid. Occasionally, one or both frontal sinuses are hypoplastic or absent.6 The arterial supply to the frontal sinuses is through the supraorbital and supratrochlear branches of the ophthalmic artery as well as the anterior ethmoidal artery (also supplying anterior ethmoid air cells), a branch of the ophthalmic artery.6,11 Their venous drainage is primarily through the superior ophthalmic veins. The primary lymphatic drainage of the frontal sinuses is to the submandibular (level IB) lymph nodes. Innervation is mainly through the supraorbital branch of the frontal nerve (a distal branch of the ophthalmic [V1] division of the trigeminal nerve). The ethmoid sinuses or air cells are formed of numerous thin-walled spaces within the ethmoidal labyrinth, varying in number from 3 to 18 per side. These cells are located between the superolateral margin of the nasal cavity and the medial aspect of the orbit, separated from the latter by a thin osseous plate, the lamina papyracea. Given this relationship, the presence of defects such as from prior lamina papyracea fractures or developmental defects or dehiscence,33 particularly those with herniation of orbital fat into the sinus ( The ethmoid air cells are functionally divided into anterior and posterior groups by the basal lamella of the middle turbinate as discussed earlier ( Fig. 1.13 Axial image from a sinus CT demonstrating an example of an old left lamina papyracea fracture with mild herniation of extraconal fat into the ethmoidal labyrinth (arrow). The anterior ethmoid air cells constitute approximately two-thirds to three quarters of the ethmoid air cells.6,34 The ethmoid bulla is usually the largest and most constant anterior ethmoid air cell ( Ethmoid air cells may pneumatize the frontal process of the maxilla adjacent to the anterior attachment of the middle turbinate to the ethmoid crest of the ascending process of the maxilla. These are known as agger nasi cells ( An ethmoid air cell may invade the medial orbital floor, known as a Haller’s cell or infraorbital ethmoid cell ( Fig. 1.14 Frontal sinus drainage pathway (FSDP) or recess. Sagittal reformat from a sinus CT demonstrates an example of the FSDP. The FSDP has a superior compartment communicating with the frontal sinus ostium (between red arrows) and a narrower inferior compartment communicating with the middle meatus. Depending on the anatomy and site of insertion of the uncinate process anteriorly, the inferior compartment may either be formed by the ethmoid infundibulum or by the middle meatus. In the current example, it is formed by the ethmoid infundibulum (red dotted line). This occurs when the anterior portion of the uncinate process extends superiorly and attaches to the skull base. In these cases, the ethmoid infundibulum drains into the middle meatus via the hiatus semilunaris. On the other hand, in cases where the anterior part of the uncinate process attaches to the lamina papyracea instead of the skull base, the inferior compartment is formed by the middle meatus itself (not shown). The basal lamella of the middle turbinate, separating anterior from posterior ethmoidal air cells, is marked by the blue dotted line. AN, agger nasi cell; FS, frontal sinus. Table 1.1 Types of air cells around the frontal sinus drainage pathway or recess
1.1 Introduction
1.2 Embryological Development
1.2.1 Development of the Anterior Skull Base
1.2.2 Development of the Nasal Cavity and Paranasal Sinuses
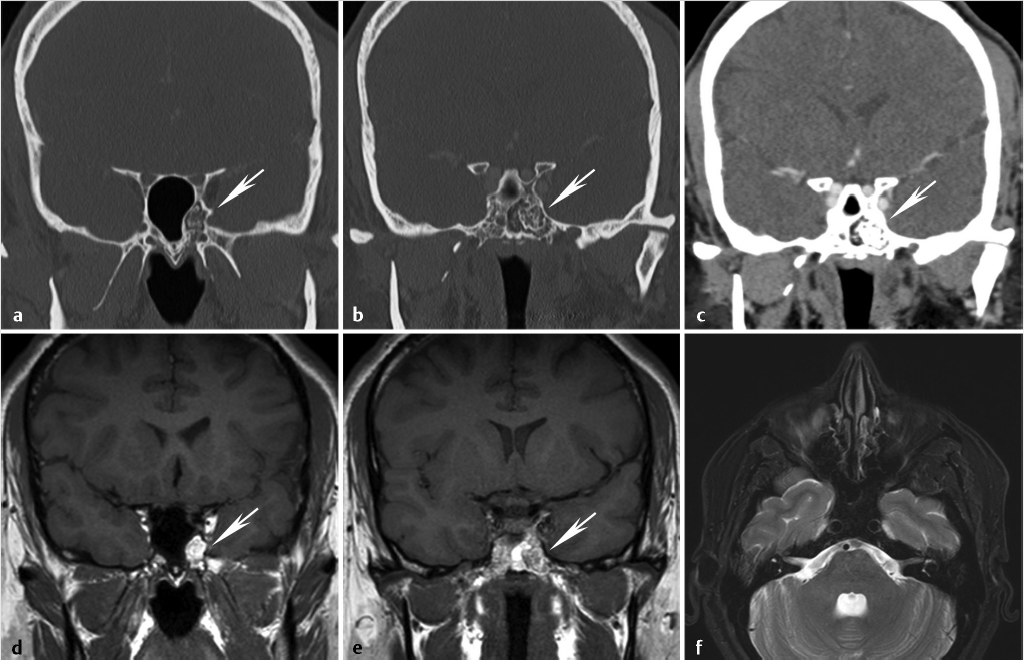
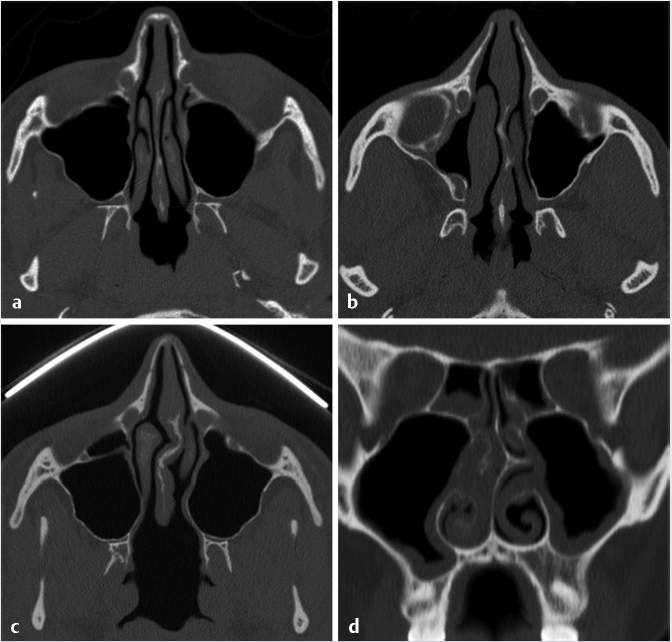
 Fig. 1.1).
Fig. 1.1).
1.3 Anatomy Overview: Applied Anatomy and Clinically Relevant Variants
1.3.1 Anterior Skull Base
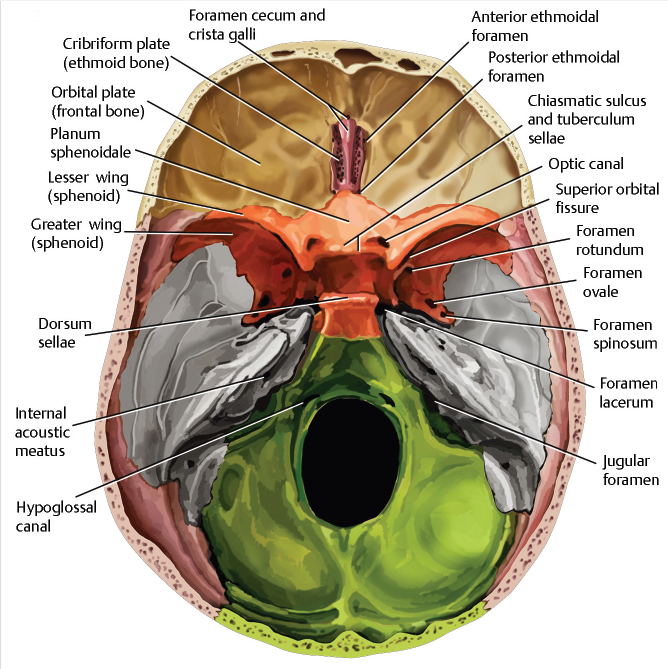
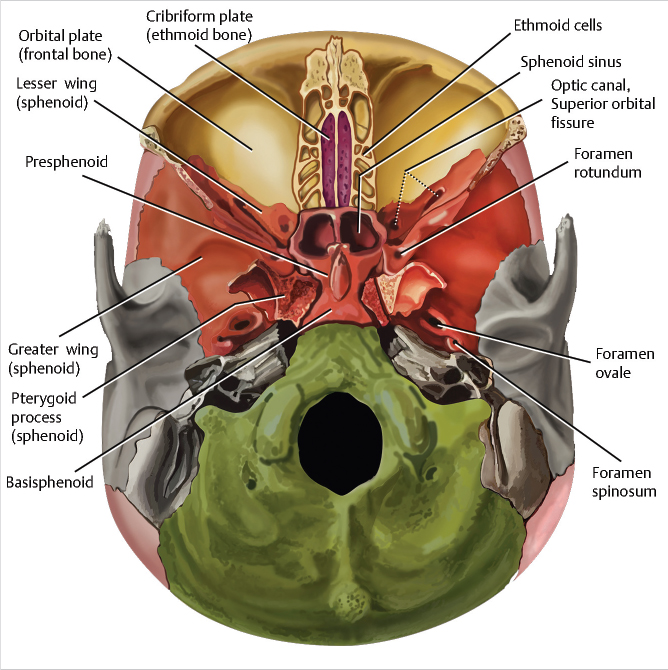
 Fig. 1.2,
Fig. 1.2,  Fig. 1.3). More posteriorly, the anterior skull base is formed by part of the body (planum sphenoidale) and lesser wing of the sphenoid bone (
Fig. 1.3). More posteriorly, the anterior skull base is formed by part of the body (planum sphenoidale) and lesser wing of the sphenoid bone ( Fig. 1.2,
Fig. 1.2,  Fig. 1.3). The planum sphenoidale, also sometimes referred to as the jugum sphenoidale, refers to the central plane of the intracranial surface of the sphenoid bone that is anterior to the sella turcica and in between the lesser wings of the sphenoid on either side (
Fig. 1.3). The planum sphenoidale, also sometimes referred to as the jugum sphenoidale, refers to the central plane of the intracranial surface of the sphenoid bone that is anterior to the sella turcica and in between the lesser wings of the sphenoid on either side ( Fig. 1.2). The anterior clinoid processes are formed by the medial part of the lesser wings of the sphenoid and serve as sites of attachment for the anterior part of the tentorium cerebelli anteriorly. The anterolateral boundary of the anterior skull base is formed by the frontal bones. The posterior margin of planum sphenoidale (tuberculum sellae, medially) and the lesser wing of the sphenoid forming the sphenoid ridge (laterally) separate the anterior skull base from the central skull base posteriorly (
Fig. 1.2). The anterior clinoid processes are formed by the medial part of the lesser wings of the sphenoid and serve as sites of attachment for the anterior part of the tentorium cerebelli anteriorly. The anterolateral boundary of the anterior skull base is formed by the frontal bones. The posterior margin of planum sphenoidale (tuberculum sellae, medially) and the lesser wing of the sphenoid forming the sphenoid ridge (laterally) separate the anterior skull base from the central skull base posteriorly ( Fig. 1.2).
Fig. 1.2).
 Fig. 1.2). The crista galli is a midline triangular process arising from the ethmoid, also a site of attachment for the falx cerebri (
Fig. 1.2). The crista galli is a midline triangular process arising from the ethmoid, also a site of attachment for the falx cerebri ( Fig. 1.2,
Fig. 1.2,  Fig. 1.4). The crista galli can be pneumatized in approximately 2 to 14% of cases in different studies12,13,14 (
Fig. 1.4). The crista galli can be pneumatized in approximately 2 to 14% of cases in different studies12,13,14 ( Fig. 1.4). Although pneumatization of this structure represents an anatomic variant, there can be inflammation of the pneumatized crista galli similar to paranasal sinuses and there are even case reports of mucocele formation in this area.15,16 It has been suggested that the primary origin of the pneumatization of the crista galli is from the frontal sinus.13
Fig. 1.4). Although pneumatization of this structure represents an anatomic variant, there can be inflammation of the pneumatized crista galli similar to paranasal sinuses and there are even case reports of mucocele formation in this area.15,16 It has been suggested that the primary origin of the pneumatization of the crista galli is from the frontal sinus.13
 Fig. 1.2,
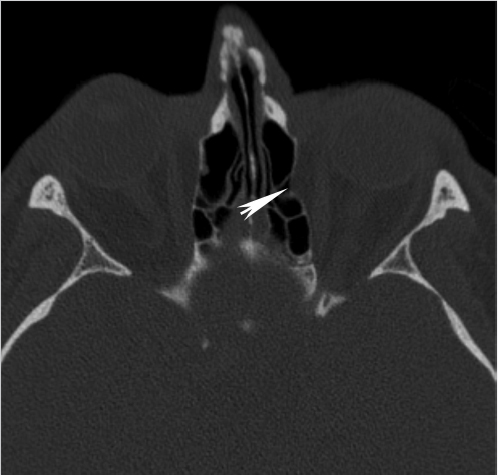
Fig. 1.2,  Fig. 1.3). The cribriform plate contains multiple small perforations that transmit afferent fibers from the nasal mucosa to the olfactory bulbs. At the junction of the ethmoid (cribriform plate) and the adjacent frontal bone, the frontoethmoidal suture contains the foramen cecum, corresponding to a small midline pit anterior to the crista galli. The data on the development, patency, and presence of a functional vein through the foramen cecum is sparse. However, although absent in the majority of the population, there can be rare instances of a patent small emissary vein in children and rarely in adults that interconnects the venous system of the nasal cavity and the superior sagittal sinus.17,18 When patent, this may represent a potential route for spread of infection/inflammation or rarely be associated with cerebrospinal fluid (CSF) leaks.17,18,19
Fig. 1.3). The cribriform plate contains multiple small perforations that transmit afferent fibers from the nasal mucosa to the olfactory bulbs. At the junction of the ethmoid (cribriform plate) and the adjacent frontal bone, the frontoethmoidal suture contains the foramen cecum, corresponding to a small midline pit anterior to the crista galli. The data on the development, patency, and presence of a functional vein through the foramen cecum is sparse. However, although absent in the majority of the population, there can be rare instances of a patent small emissary vein in children and rarely in adults that interconnects the venous system of the nasal cavity and the superior sagittal sinus.17,18 When patent, this may represent a potential route for spread of infection/inflammation or rarely be associated with cerebrospinal fluid (CSF) leaks.17,18,19
1.3.2 Nasal Cavity and Paranasal Sinuses
Nasal Cavity
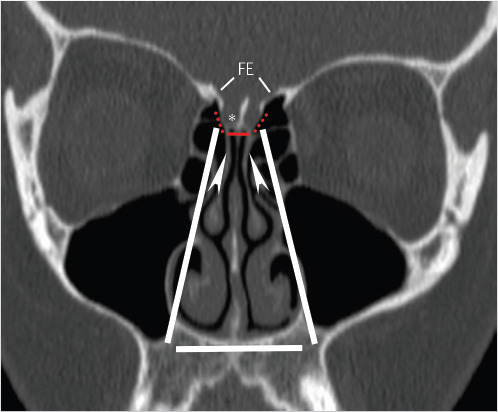
 Fig. 1.5). The horizontal central part of the roof of the nasal cavity is formed by the cribriform plate of the ethmoid bone (
Fig. 1.5). The horizontal central part of the roof of the nasal cavity is formed by the cribriform plate of the ethmoid bone ( Fig. 1.5), separating the nasal cavity from the anterior cranial fossa. Posteriorly, the nasal cavity roof is formed by the anterior aspect of the body of the sphenoid and the superior turbinates, with bilateral interruptions for the sphenoid sinus ostia. Anteriorly, the nasal cavity roof slopes down along the inner aspect of the nasal bones and nasal spine of the frontal bone.
Fig. 1.5), separating the nasal cavity from the anterior cranial fossa. Posteriorly, the nasal cavity roof is formed by the anterior aspect of the body of the sphenoid and the superior turbinates, with bilateral interruptions for the sphenoid sinus ostia. Anteriorly, the nasal cavity roof slopes down along the inner aspect of the nasal bones and nasal spine of the frontal bone.
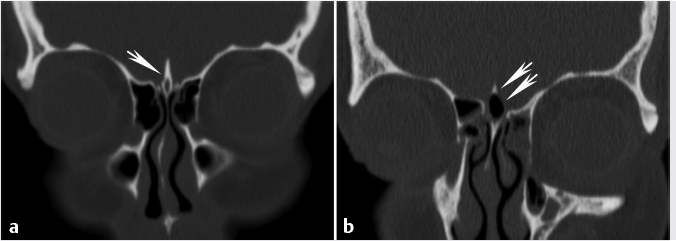
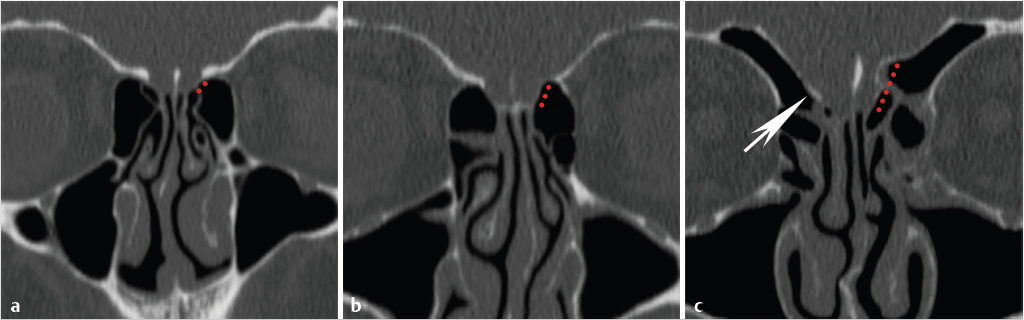
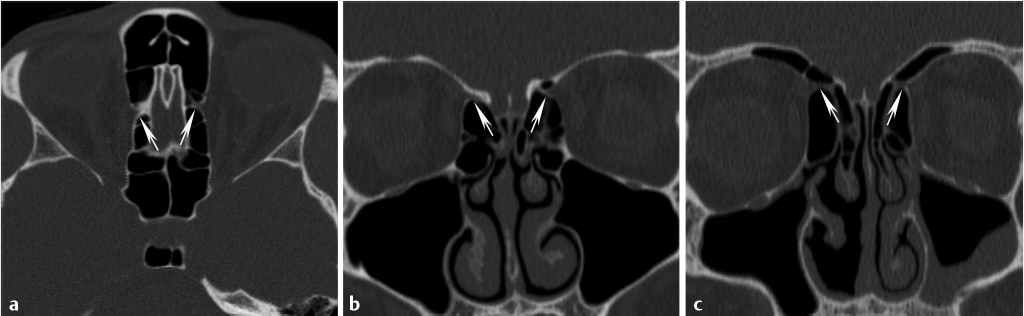
 Fig. 1.5).20 The lateral lamella of the cribriform plate is the thinnest structure in the skull base and as such represents a site of potential breach or injury, vulnerable to surgical trauma during exenteration of disease from the anterior ethmoid air cells in the area of the superior attachment of the middle turbinate.21,22,23 It connects to the fovea ethmoidalis, the roof of the ethmoid labyrinth that is formed by the orbital plate of the frontal bone. In a classic study in 1962, Keros classified the depth of the olfactory fossa, as defined by the height of the lateral lamina of the cribriform plate, into three categories. Type 1 corresponds to a depth of 1 to 3 mm, type 2 to a depth of 4 to 7 mm (most common), and type 3 to a depth of 8 to 16 mm (
Fig. 1.5).20 The lateral lamella of the cribriform plate is the thinnest structure in the skull base and as such represents a site of potential breach or injury, vulnerable to surgical trauma during exenteration of disease from the anterior ethmoid air cells in the area of the superior attachment of the middle turbinate.21,22,23 It connects to the fovea ethmoidalis, the roof of the ethmoid labyrinth that is formed by the orbital plate of the frontal bone. In a classic study in 1962, Keros classified the depth of the olfactory fossa, as defined by the height of the lateral lamina of the cribriform plate, into three categories. Type 1 corresponds to a depth of 1 to 3 mm, type 2 to a depth of 4 to 7 mm (most common), and type 3 to a depth of 8 to 16 mm ( Fig. 1.6). The height of the lateral lamella and corresponding depth of the olfactory fossa is directly related to the surgical risk of intracranial entry24 and iatrogenic CSF leak following endoscopic endonasal surgery for benign and malignant disease. Therefore, the greater the height or depth, the greater the risk. It should be noted that in addition to the lateral lamella of the cribriform plate, the anterior part of the ethmoid roof, in the area just behind the frontal recess, also represents a site of potential inadvertent breach during endoscopic surgery.25
Fig. 1.6). The height of the lateral lamella and corresponding depth of the olfactory fossa is directly related to the surgical risk of intracranial entry24 and iatrogenic CSF leak following endoscopic endonasal surgery for benign and malignant disease. Therefore, the greater the height or depth, the greater the risk. It should be noted that in addition to the lateral lamella of the cribriform plate, the anterior part of the ethmoid roof, in the area just behind the frontal recess, also represents a site of potential inadvertent breach during endoscopic surgery.25
 Fig. 1.7). The bony nasal septum is formed by the perpendicular plate of the ethmoid bone superiorly (descending from the cribriform plate) and the vomer inferiorly. The shape of the nasal septum is highly variable and, frequently, there is some degree of deviation from the midline. The septum can assume an S-shaped configuration and bony spurs may project from the septum (
Fig. 1.7). The bony nasal septum is formed by the perpendicular plate of the ethmoid bone superiorly (descending from the cribriform plate) and the vomer inferiorly. The shape of the nasal septum is highly variable and, frequently, there is some degree of deviation from the midline. The septum can assume an S-shaped configuration and bony spurs may project from the septum ( Fig. 1.7).
Fig. 1.7).
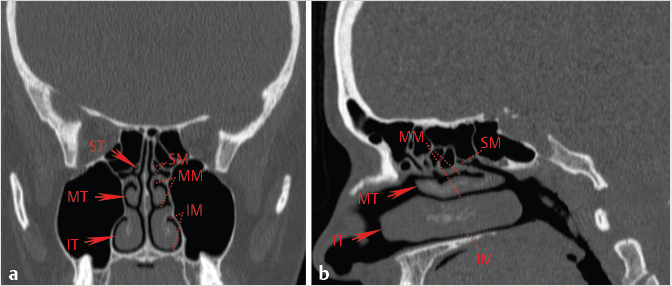
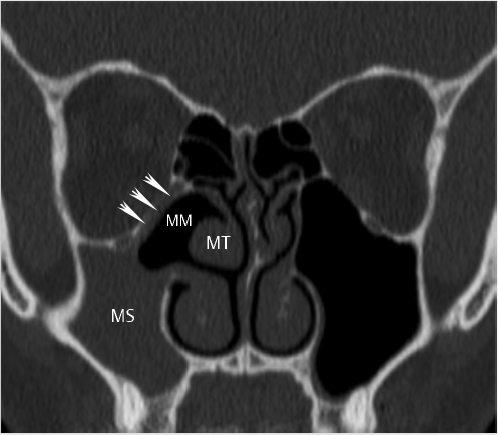
 Fig. 1.8). Some, but not all, individuals also have small supreme turbinates (arising from the ethmoid bone), but these have little clinical or radiologic significance. The nasal concha/turbinates curve inferomedially and form a roof for a groove, or meatus. As such, the meati are the nasal cavity airspaces inferior and lateral to their respective turbinates (
Fig. 1.8). Some, but not all, individuals also have small supreme turbinates (arising from the ethmoid bone), but these have little clinical or radiologic significance. The nasal concha/turbinates curve inferomedially and form a roof for a groove, or meatus. As such, the meati are the nasal cavity airspaces inferior and lateral to their respective turbinates ( Fig. 1.8). These airspaces represent the ultimate site of drainage of the paranasal sinuses as well as the nasolacrimal duct.
Fig. 1.8). These airspaces represent the ultimate site of drainage of the paranasal sinuses as well as the nasolacrimal duct.
 Fig. 1.8). The superior turbinate is an important landmark during endoscopic surgery for the identification of the sphenoid sinus and its natural ostium. In a majority of patients, the sphenoid sinus ostium is located medial relative to the posterior part of the superior turbinate, between the superior turbinate and nasal septum. In a very small percentage of patients (2% in one study), the natural ostium can be located laterally.26
Fig. 1.8). The superior turbinate is an important landmark during endoscopic surgery for the identification of the sphenoid sinus and its natural ostium. In a majority of patients, the sphenoid sinus ostium is located medial relative to the posterior part of the superior turbinate, between the superior turbinate and nasal septum. In a very small percentage of patients (2% in one study), the natural ostium can be located laterally.26
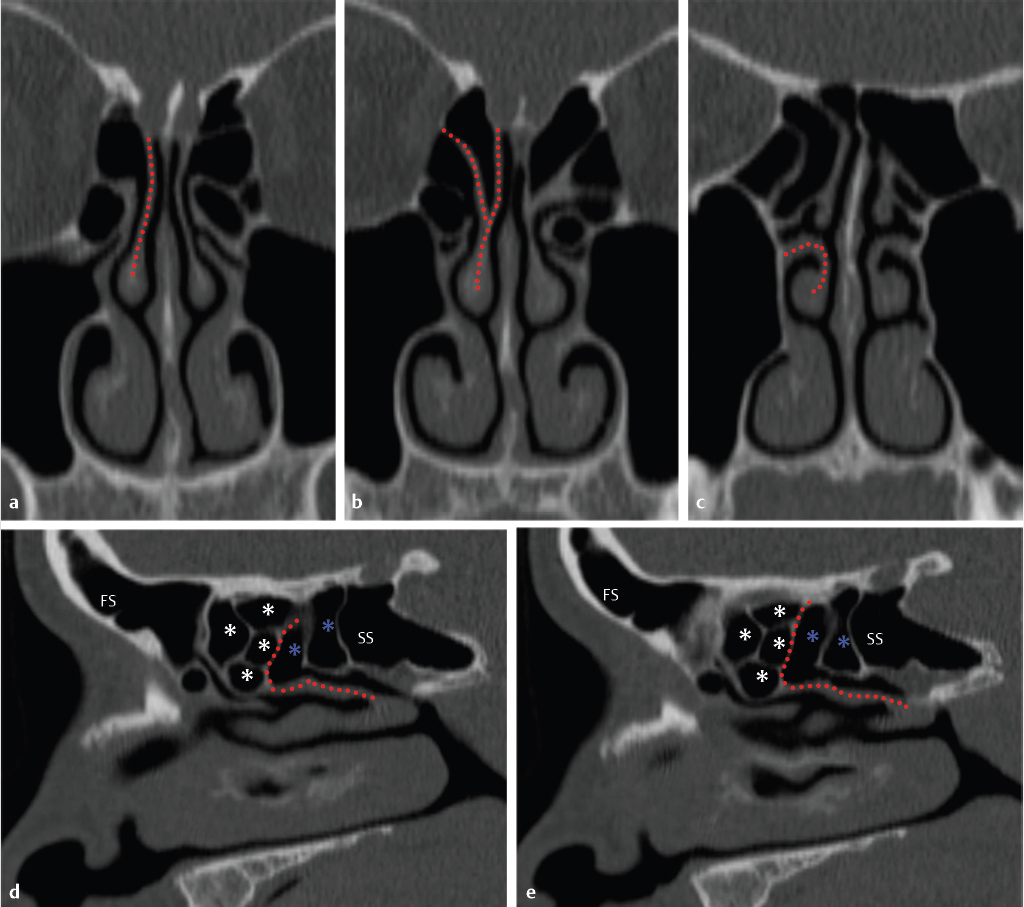
 Fig. 1.9). There is variation in the terminology used which can cause confusion, but according to some descriptions, the basal lamella of the middle turbinate can be divided into three portions.27,28 An anterior vertical portion lies in a near sagittal plane and attaches to the cribriform plate, between the medial and lateral lamella of the cribriform plate (
Fig. 1.9). There is variation in the terminology used which can cause confusion, but according to some descriptions, the basal lamella of the middle turbinate can be divided into three portions.27,28 An anterior vertical portion lies in a near sagittal plane and attaches to the cribriform plate, between the medial and lateral lamella of the cribriform plate ( Fig. 1.5,
Fig. 1.5,  Fig. 1.9). More posteriorly, the middle portion lies in a nearly coronal plane and attaches to the lamina papyracea (
Fig. 1.9). More posteriorly, the middle portion lies in a nearly coronal plane and attaches to the lamina papyracea ( Fig. 1.9). The most posterior portion lies in a nearly horizontal plane and attaches to the lamina papyracea, medial maxillary sinus wall, or both (
Fig. 1.9). The most posterior portion lies in a nearly horizontal plane and attaches to the lamina papyracea, medial maxillary sinus wall, or both ( Fig. 1.9). As would be expected, the anterior part of the basal lamella is best seen on the coronal plane, whereas the middle and posterior parts are best seen in the sagittal plane (
Fig. 1.9). As would be expected, the anterior part of the basal lamella is best seen on the coronal plane, whereas the middle and posterior parts are best seen in the sagittal plane ( Fig. 1.5,
Fig. 1.5,  Fig. 1.9). The latter are also the landmark for separation of the anterior from the posterior ethmoid air cells (
Fig. 1.9). The latter are also the landmark for separation of the anterior from the posterior ethmoid air cells ( Fig. 1.9). As discussed earlier, there is variation in the terminology used for different parts of the middle turbinate, and some consider the anterior vertical part attaching to the cribriform plate the vertical lamella and the more posterior parts the basal lamella.3
Fig. 1.9). As discussed earlier, there is variation in the terminology used for different parts of the middle turbinate, and some consider the anterior vertical part attaching to the cribriform plate the vertical lamella and the more posterior parts the basal lamella.3
 Fig. 1.10), with significant variation in extent of pneumatization seen in different patients. In one study, 53% of patients examined had some degree of middle turbinate pneumatization.29 Pneumatization may be limited to the upper vertical lamella or extend more caudally to the bulbous portion of the turbinate. There is again variation in the use of terminology, but when the pneumatization is limited to the vertical lamina, some refer to it as partial pneumatization, whereas some reserve the term concha bullosa only when the pneumatization extends to the caudal bulbous portion of the turbinate.6 Middle turbinate pneumatization is commonly an incidental finding of no clinical significance. However, some believe that concha bullosa may become clinically significant when they are large and result in obstruction of drainage pathways and air cells. Another common variation of middle turbinates is paradoxical curvature. This refers to cases where the middle turbinate has a convex curvature on its lateral side rather than the more common medial side. This is typically considered to be of no clinical significance unless it obviously obstructs an air channel.6,30 Unlike the middle turbinates, pneumatization of the inferior turbinates is a rare finding.31
Fig. 1.10), with significant variation in extent of pneumatization seen in different patients. In one study, 53% of patients examined had some degree of middle turbinate pneumatization.29 Pneumatization may be limited to the upper vertical lamella or extend more caudally to the bulbous portion of the turbinate. There is again variation in the use of terminology, but when the pneumatization is limited to the vertical lamina, some refer to it as partial pneumatization, whereas some reserve the term concha bullosa only when the pneumatization extends to the caudal bulbous portion of the turbinate.6 Middle turbinate pneumatization is commonly an incidental finding of no clinical significance. However, some believe that concha bullosa may become clinically significant when they are large and result in obstruction of drainage pathways and air cells. Another common variation of middle turbinates is paradoxical curvature. This refers to cases where the middle turbinate has a convex curvature on its lateral side rather than the more common medial side. This is typically considered to be of no clinical significance unless it obviously obstructs an air channel.6,30 Unlike the middle turbinates, pneumatization of the inferior turbinates is a rare finding.31
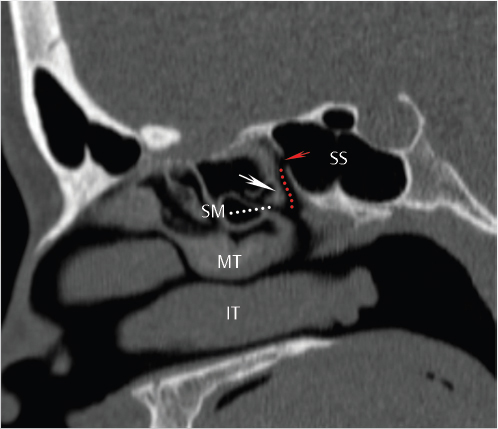
 Fig. 1.8). Posterior ethmoidal air cells drain via a variable number of ostia into the anterior portion of the superior meatus and then into the sphenoethmoidal recess (the recess between the superior concha and the body of the sphenoid;
Fig. 1.8). Posterior ethmoidal air cells drain via a variable number of ostia into the anterior portion of the superior meatus and then into the sphenoethmoidal recess (the recess between the superior concha and the body of the sphenoid;  Fig. 1.11). The sphenoethmoidal recess represents the site of drainage of the sphenoid sinus via the sphenoid ostium and leads to the superior meatus anteriorly. Drainage is ultimately into the posterior nasal cavity and nasopharynx.
Fig. 1.11). The sphenoethmoidal recess represents the site of drainage of the sphenoid sinus via the sphenoid ostium and leads to the superior meatus anteriorly. Drainage is ultimately into the posterior nasal cavity and nasopharynx.
 Fig. 1.8). It receives drainage from the frontal sinus, anterior ethmoid air cells, and the maxillary sinus (
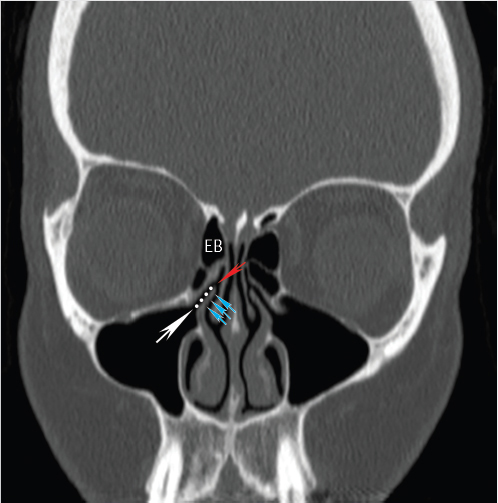
Fig. 1.8). It receives drainage from the frontal sinus, anterior ethmoid air cells, and the maxillary sinus ( Fig. 1.12; discussed in greater detail in the following sections). The middle meatus contains a rounded elevation called the bulla ethmoidalis (
Fig. 1.12; discussed in greater detail in the following sections). The middle meatus contains a rounded elevation called the bulla ethmoidalis ( Fig. 1.12), representing the curved surface covering the anterior medial ethmoid air cells.6 Inferior to this, there is a curved cleft known as the hiatus semilunaris (
Fig. 1.12), representing the curved surface covering the anterior medial ethmoid air cells.6 Inferior to this, there is a curved cleft known as the hiatus semilunaris ( Fig. 1.12). The inferior margin of the hiatus is formed by the uncinate process (
Fig. 1.12). The inferior margin of the hiatus is formed by the uncinate process ( Fig. 1.12). The ostium of the maxillary sinus is normally located lateral to the uncinate process. Pertaining to frontal sinus drainage, anatomic variations of the uncinate process determine the pathway for the drainage of the frontal sinus in this region and are discussed in greater detail later. The functional anatomy of the inferior meatal region is more straightforward. The inferior meatus is the largest of the meati (
Fig. 1.12). The ostium of the maxillary sinus is normally located lateral to the uncinate process. Pertaining to frontal sinus drainage, anatomic variations of the uncinate process determine the pathway for the drainage of the frontal sinus in this region and are discussed in greater detail later. The functional anatomy of the inferior meatal region is more straightforward. The inferior meatus is the largest of the meati ( Fig. 1.8) and the ostium of the nasolacrimal canal is located at the junction of its anterior and middle thirds.
Fig. 1.8) and the ostium of the nasolacrimal canal is located at the junction of its anterior and middle thirds.
Paranasal Sinuses
Frontal sinus
Ethmoid sinus
 Fig. 1.13), should be reported to avoid misinterpretation as inflammatory disease or unintended surgical manipulation. The smaller anterior part of the lateral ethmoid wall is formed by the lacrimal bone. In the adult, the ethmoid labyrinth is usually pyramidal shaped, with its base directed posteriorly. Therefore, it becomes wider posteriorly. Less commonly, it may have similar dimensions anteriorly and posteriorly. This is important to recognize because the operative field will not get wider as one proceeds posteriorly during surgery.
Fig. 1.13), should be reported to avoid misinterpretation as inflammatory disease or unintended surgical manipulation. The smaller anterior part of the lateral ethmoid wall is formed by the lacrimal bone. In the adult, the ethmoid labyrinth is usually pyramidal shaped, with its base directed posteriorly. Therefore, it becomes wider posteriorly. Less commonly, it may have similar dimensions anteriorly and posteriorly. This is important to recognize because the operative field will not get wider as one proceeds posteriorly during surgery.
 Fig. 1.9). Within each group, the sinuses are divided by incomplete osseous septations. Broadly, the anterior group of ethmoid air cells drain via one or more orifices into the ethmoid bulla and the hiatus semilunaris (also discussed later) and then to the middle meatus (
Fig. 1.9). Within each group, the sinuses are divided by incomplete osseous septations. Broadly, the anterior group of ethmoid air cells drain via one or more orifices into the ethmoid bulla and the hiatus semilunaris (also discussed later) and then to the middle meatus ( Fig. 1.12). More specifically, the anterior ethmoid air cells can be subdivided into frontal recess cells (draining into the frontal recess), infundibular cells (draining into the infundibulum and hiatus semilunaris), and bullae cells (draining into a groove on the bulla ethmoidalis).6 Ultimately, these drain into the middle meatus. The drainage of the posterior ethmoid air cell group typically is through the superior meatus and the sphenoethmoidal recess and then into the nasopharynx (
Fig. 1.12). More specifically, the anterior ethmoid air cells can be subdivided into frontal recess cells (draining into the frontal recess), infundibular cells (draining into the infundibulum and hiatus semilunaris), and bullae cells (draining into a groove on the bulla ethmoidalis).6 Ultimately, these drain into the middle meatus. The drainage of the posterior ethmoid air cell group typically is through the superior meatus and the sphenoethmoidal recess and then into the nasopharynx ( Fig. 1.11).
Fig. 1.11).
 Fig. 1.12), although it can vary significantly in extent of pneumatization. If very large, this could theoretically impinge on the ostiomeatal unit (OMU), although typically it is incidental and it is not clear whether this can have a true effect or relation to sinusitis. As discussed earlier, there can also be pneumatization of the middle turbinate, and some have proposed that when large enough a concha bullosa (
Fig. 1.12), although it can vary significantly in extent of pneumatization. If very large, this could theoretically impinge on the ostiomeatal unit (OMU), although typically it is incidental and it is not clear whether this can have a true effect or relation to sinusitis. As discussed earlier, there can also be pneumatization of the middle turbinate, and some have proposed that when large enough a concha bullosa ( Fig. 1.10) may result in sinus obstruction. However, the majority of time these are incidental and the association with sinusitis is not clear-cut.35
Fig. 1.10) may result in sinus obstruction. However, the majority of time these are incidental and the association with sinusitis is not clear-cut.35
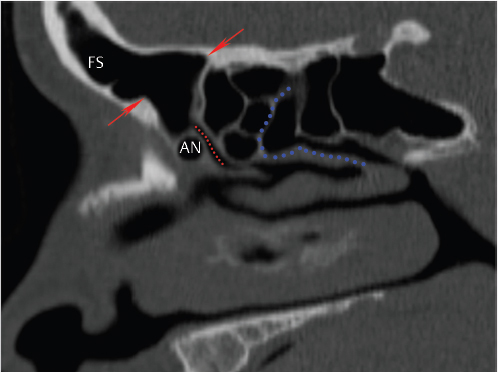
 Fig. 1.14). The reported incidence of these cells varies considerably between different studies (3–100%).6,36 When present, these are the most anterior ethmoid air cells, located anterior, inferior, and lateral to the frontal recess. They are deep to the lacrimal bone and also serve as a landmark for the nasofrontal duct. The majority of time, these are of no clinical significance, although some have suggested a potential association with frontal sinusitis.36,37 Anterior ethmoid air cells may pneumatize the roof of the orbits, known as supraorbital ethmoid cells (
Fig. 1.14). The reported incidence of these cells varies considerably between different studies (3–100%).6,36 When present, these are the most anterior ethmoid air cells, located anterior, inferior, and lateral to the frontal recess. They are deep to the lacrimal bone and also serve as a landmark for the nasofrontal duct. The majority of time, these are of no clinical significance, although some have suggested a potential association with frontal sinusitis.36,37 Anterior ethmoid air cells may pneumatize the roof of the orbits, known as supraorbital ethmoid cells ( Table 1.1). On axial and sagittal images, these are posterior to and separated from the frontal sinus by an intact wall. Preoperative identification of these cells is important to avoid mistaking them for the frontal ostium during endoscopic surgery.34
Table 1.1). On axial and sagittal images, these are posterior to and separated from the frontal sinus by an intact wall. Preoperative identification of these cells is important to avoid mistaking them for the frontal ostium during endoscopic surgery.34
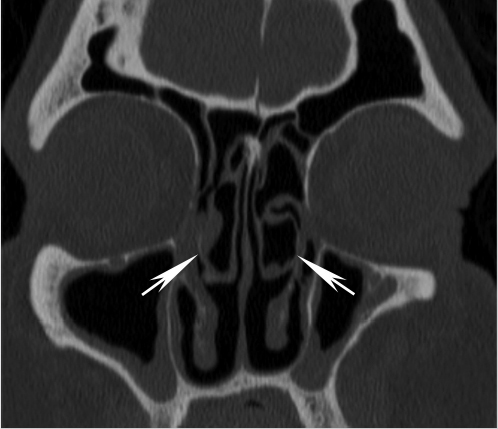
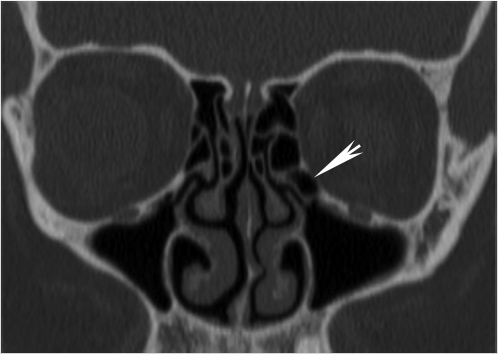
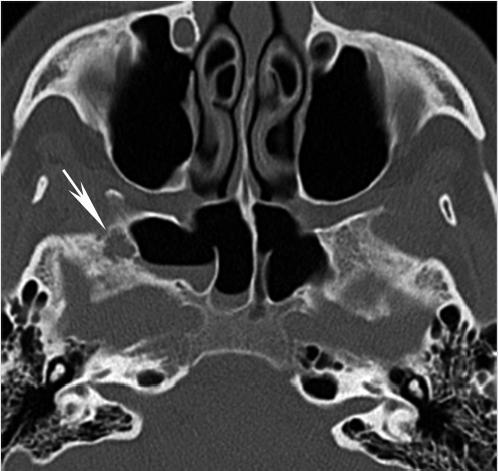
 Fig. 1.15). Some believe that Haller’s cells may contribute to rhinosinusitis by narrowing the infundibulum, but there is controversy and inconsistent reports regarding this association.36,38,39,40 Regardless, awareness of this variant is important because its presence can increase the risk of intraorbital injury during surgery.36,39,40 A posterior ethmoid air cell may also extend posterior to the maxillary sinus, resulting in a double maxillary antrum. If that cell is infected, this must be recognized because it will require opening of the posterior maxillary sinus wall for access to that cell.
Fig. 1.15). Some believe that Haller’s cells may contribute to rhinosinusitis by narrowing the infundibulum, but there is controversy and inconsistent reports regarding this association.36,38,39,40 Regardless, awareness of this variant is important because its presence can increase the risk of intraorbital injury during surgery.36,39,40 A posterior ethmoid air cell may also extend posterior to the maxillary sinus, resulting in a double maxillary antrum. If that cell is infected, this must be recognized because it will require opening of the posterior maxillary sinus wall for access to that cell.
Type | Description |
Supraorbital ethmoid cell | Anterior ethmoid air cell that extends superiorly and laterally over the orbit from the frontal recess and drain into the frontal recess; separated from the frontal sinus by a bony septum; it should not be mistaken for a septated frontal sinus |
Frontal cells | Type 1: Single air cell above the agger nasi cell that does not extend into the frontal sinus |
Suprabullar cell | Air cell above the ethmoid bulla whose anterior wall does not extend into the frontal sinus |
Frontal bullar cell | Air cell above the ethmoid bulla that extends into the frontal sinus |
Inter–frontal sinus septal cell | Pneumatized frontal sinus septum |
Source: Modified from Huang et al34
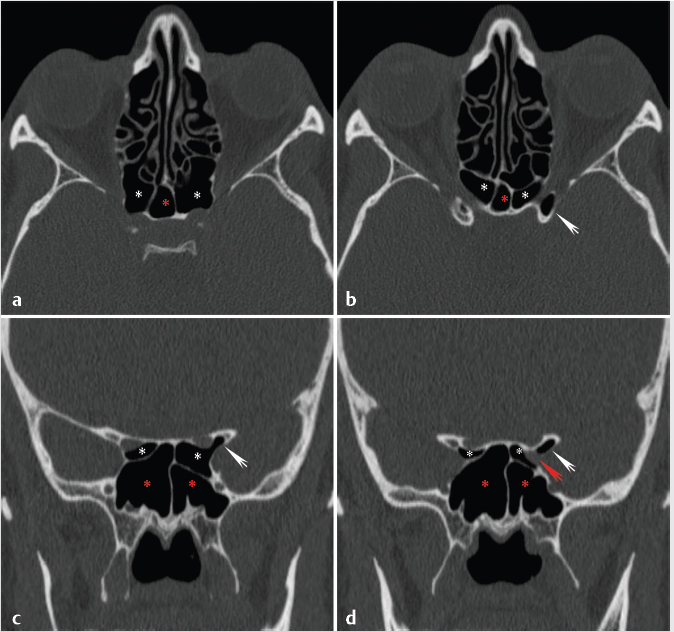
Posterior ethmoid cells can also extend into the sphenoid sinus, typically located superiorly and laterally and partly surrounding the optic nerve canal. This is referred to as an Onodi cell ( Fig. 1.16). The importance of the Onodi cell is that there is potential for misidentification during endoscopic surgery. This puts closely related critical structures, in particular the optic nerves, at risk for inadvertent surgical injury.39,41,42 Sphenoid septations are typically not in the horizontal plane and the presence of horizontal or near-horizontal septations in the sphenoid area on coronal images suggests the presence of Onodi cells. These can be present unilaterally or bilaterally and may coexist with other anatomic variations of importance (
Fig. 1.16). The importance of the Onodi cell is that there is potential for misidentification during endoscopic surgery. This puts closely related critical structures, in particular the optic nerves, at risk for inadvertent surgical injury.39,41,42 Sphenoid septations are typically not in the horizontal plane and the presence of horizontal or near-horizontal septations in the sphenoid area on coronal images suggests the presence of Onodi cells. These can be present unilaterally or bilaterally and may coexist with other anatomic variations of importance ( Fig. 1.16). Some reports describe the sphenomaxillary plate, referring to a thin bony partition between the maxillary sinus and the sphenoid sinus.42 If present, there is the potential for the sphenoid sinus to be mistaken for posterior ethmoid cells during the transantral ethmoidectomy.42
Fig. 1.16). Some reports describe the sphenomaxillary plate, referring to a thin bony partition between the maxillary sinus and the sphenoid sinus.42 If present, there is the potential for the sphenoid sinus to be mistaken for posterior ethmoid cells during the transantral ethmoidectomy.42
As discussed earlier, the roof of the ethmoid labyrinth is formed by the orbital plate of the frontal bone and is referred to as the fovea ethmoidalis. The Keros’s classification for cribriform plate and ethmoid roof configuration was discussed earlier ( Fig. 1.6) and it is worth emphasizing that the fovea ethmoidalis is at a slight angle and descends as it extends posteriorly. Therefore, anteriorly, it can be higher than the cribriform plates (
Fig. 1.6) and it is worth emphasizing that the fovea ethmoidalis is at a slight angle and descends as it extends posteriorly. Therefore, anteriorly, it can be higher than the cribriform plates ( Fig. 1.5,
Fig. 1.5,  Fig. 1.6). In addition to its depth, it is also important to note any ethmoid roof asymmetry as this predisposes to intracranial penetration during endoscopic sinus surgery, usually on the side where the ethmoid roof is lower (
Fig. 1.6). In addition to its depth, it is also important to note any ethmoid roof asymmetry as this predisposes to intracranial penetration during endoscopic sinus surgery, usually on the side where the ethmoid roof is lower ( Fig. 1.6).43 However, other than absolute differences in height, asymmetry in shape, including a flat sloping shape of ethmoid roof (
Fig. 1.6).43 However, other than absolute differences in height, asymmetry in shape, including a flat sloping shape of ethmoid roof ( Fig. 1.6), may also predispose to skull base injury.44
Fig. 1.6), may also predispose to skull base injury.44
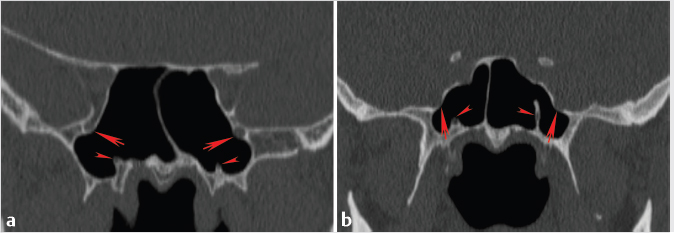
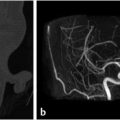
Other anatomic structures worth evaluating during preoperative planning are the canals for the anterior and posterior ethmoidal arteries ( Fig. 1.17). The anterior ethmoidal artery crosses from the orbit through the ethmoidal labyrinth and through the lateral lamella of the cribriform plate at the anterior ethmoidal sulcus and can serve as a surgical landmark.45 In addition, this is the area where the bone is thinnest in the anterior skull base. There are also close adhesions of dura mater to the sinus walls in the anterior ethmoidal region. As such, this is considered a high-risk area.45 In some patients, the canals for the anterior ethmoidal arteries may travel below the ethmoid roof (
Fig. 1.17). The anterior ethmoidal artery crosses from the orbit through the ethmoidal labyrinth and through the lateral lamella of the cribriform plate at the anterior ethmoidal sulcus and can serve as a surgical landmark.45 In addition, this is the area where the bone is thinnest in the anterior skull base. There are also close adhesions of dura mater to the sinus walls in the anterior ethmoidal region. As such, this is considered a high-risk area.45 In some patients, the canals for the anterior ethmoidal arteries may travel below the ethmoid roof ( Fig. 1.17). Some refer to this configuration as the canal being on a mesentery. This results in increased exposure of the artery and increases the risk of an iatrogenic injury during endoscopic sinus surgery that could lead to hematoma, including intraorbital hematoma, CSF leak, or infection.23
Fig. 1.17). Some refer to this configuration as the canal being on a mesentery. This results in increased exposure of the artery and increases the risk of an iatrogenic injury during endoscopic sinus surgery that could lead to hematoma, including intraorbital hematoma, CSF leak, or infection.23
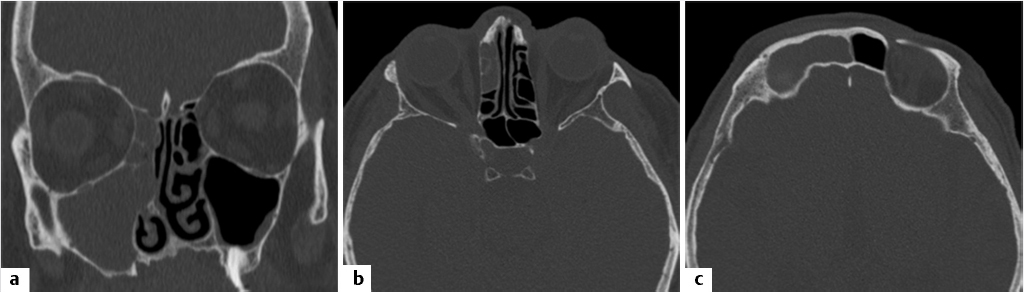
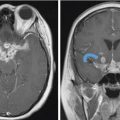
Fig. 1.16 Onodi cell configuration and other important anatomic variations of posterior ethmoidal air cells and the sphenoid sinus. (a,b) Axial and (c,d) coronal reformats from a sinus CT demonstrate multiple important variants in the same patient. In this case, there is bilateral Onodi cell configuration (white asterisks), representing extension of posterior ethmoid cells into the sphenoid sinus (red asterisks). Note the resultant exposure of optic nerves, putting them at risk for inadvertent surgical injury. In this case, this is further exacerbated by anterior clinoid pneumatization, asymmetric on the left (white arrows). There is in addition thinning and dehiscence of parts of the bony covering of the optic (c) and carotid (a,d) canals. In this particular case, the combination of anatomic variants also results in asymmetric exposure of the left carotid canal (red arrow; d).
The posterior ethmoidal artery travels along the roof of the posterior ethmoid sinus. This artery usually courses within the bony roof that is thicker than that of the anterior ethmoid sinus. However, occasionally although much less commonly than with the anterior ethmoidal artery, this artery may also course below the roof and if so, this should be noted. Because it tends to be larger, damage to this artery may cause more bleeding than damage to the anterior ethmoidal artery.23
The arterial supply to the ethmoid derives from the nasal branches of the sphenopalatine artery as well as the anterior and posterior ethmoidal arterial branches from the ophthalmic artery. As such, the arterial supply is both from branches of the internal and external carotid arteries. The venous drainage of the ethmoid air cells is through the nasal veins into the nose or through the ethmoidal veins into the ophthalmic veins. Thrombophlebitis of these valveless veins may result in cavernous sinus thrombosis as a complication of ethmoid sinusitis. Lymphatics follow the functional anterior and posterior mucociliary drainage divisions, leading to the submandibular (level IB) and retropharyngeal lymph node groups,11 respectively. Sensory innervation of the anterior ethmoid air cells is via the anterior ethmoidal nerve (arising from the nasociliary branch of the ophthalmic [V1] division of the trigeminal nerve). The posterior ethmoid air cells are innervated by branches of the posterior ethmoidal nerve (arising from ophthalmic division [V1] of the trigeminal nerve) and the posterolateral nasal branches of the sphenopalatine nerve (arising from the maxillary division [V2] of the trigeminal nerve).6
Maxillary sinus
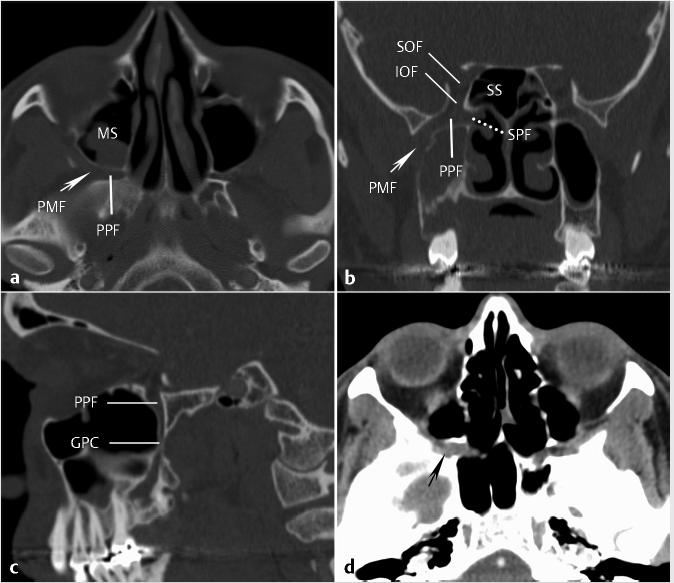
The maxillary sinuses or antra are the largest of the paranasal sinuses, filling the bodies of the maxilla. They have roughly pyramidal shapes with bases directed medially, paralleling the lateral walls of the nasal cavities. The floors are formed by the maxillary alveolar and palatine processes. The roofs of the maxillary sinuses are composed of the orbital floors and are traversed by the infraorbital groove posteriorly. The groove becomes a canal more anteriorly. Occasionally, parts of these canals may project within the sinus or in a partial septation in the sinus. Laterally, the apex of the pyramidal configuration is capped by the zygomatic process of the maxilla. The anterior superior alveolar nerve and associated vessels pass inferiorly from the infraorbital foramen resulting in a groove in the anterior wall of the sinus. Posterolaterally, the wall of the sinus abuts retromaxillary fat and contains a canal for the posterosuperior alveolar nerve to the molar dentition. The posterior wall of the maxillary sinus forms the anterior margin of the pterygopalatine fossa (PPF), discussed in greater detail later.
Most commonly, the maxillary sinuses develop symmetrically with minor common variations of unilateral or bilateral hypoplasia present in less than 10% of cases.6 Maxillary sinus hypoplasia should not be confused with an atelectatic sinus, discussed later in the section on the OMU. Other variants that may be seen include internal septa. If the septum results in complete compartmentalization, this should be noted and the site of drainage (e.g., through an accessory maxillary ostium) should be identified. Compartmentalization by extension of an ethmoid air cell posterior to the maxillary sinus was discussed earlier. Dental roots may form conical elevations in the floor of the maxillary sinus. Less commonly, dental roots may project into the antrum. Occasionally, the overlying bone may be dehiscent only with sinus mucosa separating the roots from the sinus cavity.
Medially, the maxillary bone has a large hole or defect called the maxillary hiatus. However, this defect is partially covered by parts of the ethmoid bone, the perpendicular plate of the palatine bone, the lacrimal bone, and the inferior turbinate. The main drainage of the maxillary sinus is through the maxillary ostium medially opening into the infundibulum ( Fig. 1.12; also discussed in greater detail later in the section on paranasal sinus drainage). The location of the main, natural maxillary sinus ostium can vary but is typically high, just below the floor of the orbit. Depending on the specific location, the surgeon may elect to enter the sinus at a lower level.
Fig. 1.12; also discussed in greater detail later in the section on paranasal sinus drainage). The location of the main, natural maxillary sinus ostium can vary but is typically high, just below the floor of the orbit. Depending on the specific location, the surgeon may elect to enter the sinus at a lower level.
Between the inferior part of the uncinate process and the insertion of the inferior turbinate, the medial maxillary hiatus is covered by opposing nasal and sinus mucosa. This membranous area is referred to as the fontanelle and is divided into a posterior and an anterior fontanelle by the ethmoidal process of the inferior turbinate, which extends superiorly to contact the uncinate process.6 This membranous area can break down and result in the formation of accessory maxillary ostia. In cases where the natural maxillary ostium cannot be cannulated because of a large uncinate process or because it would put the orbit at risk, this represents an alternate site of penetration into the middle meatus.6,46
The arterial supply to the maxillary sinus is through the maxillary artery including the infraorbital, greater palatine, posterosuperior alveolar, and anterior superior arteries. Venous drainage is via the anterior facial vein anteriorly or the maxillary vein posteriorly. The maxillary vein connects to the pterygoid venous plexus which in turn connects to the dural venous sinuses and this represents a potential pathway for spread of infection from maxillary sinusitis to result in meningitis. The maxillary vein also joins the superficial temporal vein to form the retromandibular vein, ultimately draining into the internal and external jugular veins. The main lymphatic drainage of the maxillary sinus is into the submandibular (level IB) lymph nodes, although there can also be drainage to other nodal stations, including the lateral retropharyngeal lymph nodes.6,47 Innervation to the maxillary sinus is through multiple branches of the superior alveolar nerve (anterior, middle, posterior), the anterior palatine nerve, and the infraorbital nerve. The posterosuperior alveolar nerve pierces the posterior maxillary sinus wall and travels anteriorly and inferiorly to supply the molar teeth.6
Sphenoid sinus
The sphenoid sinuses are located within the body of the sphenoid bone, posterior to the upper nasal cavity. There is considerable variation in the degree of pneumatization on the left and right sides of the sphenoid sinus. The sphenoid sinus septum is usually midline anteriorly, aligned with the nasal septum. However, posteriorly, it frequently can deviate far to one side, creating two unequal sinus cavities. The sphenoid septations are vertical in orientation. Therefore, if horizontal septations are seen on coronal or sagittal images, then ethmoid air cell septations or Onodi cells should be suspected ( Fig. 1.16).
Fig. 1.16).
Because of extensive variation in the degree of pneumatization of the sphenoid sinus and its potential impact on preoperative planning, different classification systems have been introduced to describe the degree of pneumatization. There is no universally accepted classification system, but generally the sphenoid sinus may be classified as conchal or non-pneuma-tized (rare; describing cases where the posterior wall of the sinus lies anterior to the sella turcica), presellar (the posterior limit of the sinus cavity extends only to the anterior wall of the sella turcica), or sellar (the sinus cavity extends posterior to the anterior sella turcica wall and lies under the sella floor, such that the sella bulges into the sinus).6,48 Some may also include a fourth type, the postsellar type, referring to cases where the posterior boundary of the sinus extends to or more posterior to the posterior part of the sella, with pneumatization completely surrounding the sella.48 The conchal type is relatively rare, constituting as little as 2% of cases in some studies.48 In this group of patients, the thick bony posterior sinus wall makes the transsphenoid approach to the pituitary particularly challenging and this anatomy may be considered a relative contraindication to transsphenoidal hypophysectomy.
In addition to variable posterior pneumatization, there is also variable lateral pneumatization and extent of the sphenoid sinus. There can be extension of the lateral recesses from the main sphenoid sinus cavity into the greater sphenoid wing, where it forms the floor of the middle cranial fossa and the posterior orbital wall, the lesser sphenoid wing, or the pterygoid process. When present, the lateral extension almost always goes between the foramen rotundum and the Vidian canal ( Fig. 1.18). Therefore, depending on sphenoid pneumatization, the foramen rotundum may either be completely outside the sinus or bulge into the lower lateral sinus wall (
Fig. 1.18). Therefore, depending on sphenoid pneumatization, the foramen rotundum may either be completely outside the sinus or bulge into the lower lateral sinus wall ( Fig. 1.18). The Vidian canal may be within the sphenoid bone proper, bulge into the sphenoid sinus, or occasionally be elevated on a septum within the sinus cavity (
Fig. 1.18). The Vidian canal may be within the sphenoid bone proper, bulge into the sphenoid sinus, or occasionally be elevated on a septum within the sinus cavity ( Fig. 1.18). The combination of prominent arachnoid granulations and widely pneumatized, thin adjacent bone could predispose to an osteodural defect resulting in a CSF leak (
Fig. 1.18). The combination of prominent arachnoid granulations and widely pneumatized, thin adjacent bone could predispose to an osteodural defect resulting in a CSF leak ( Fig. 1.19). Therefore, when evaluating CT scans for CSF leaks, these areas should be carefully scrutinized.
Fig. 1.19). Therefore, when evaluating CT scans for CSF leaks, these areas should be carefully scrutinized.
Awareness of the anatomic variations and configurations that could predispose to optic nerve or carotid injury during endoscopic sinus surgery is essential for preoperative planning and well demonstrated on high-resolution CT scans of the sinuses. One such variation is the Onodi cell, discussed earlier in the section on the ethmoid sinus ( Fig. 1.16). However, there are other anatomic configurations that may result in increased exposure of the optic nerves to injury. This includes the extent of protrusion of the optic nerves into the sphenoid sinus, classified by DeLano et al into four types (
Fig. 1.16). However, there are other anatomic configurations that may result in increased exposure of the optic nerves to injury. This includes the extent of protrusion of the optic nerves into the sphenoid sinus, classified by DeLano et al into four types ( Table 1.2).49 Regardless of whether the actual classification is used in routine clinical practice, awareness of these variations and potential exposure of the optic nerve by the otolaryngologist on preoperative scans is essential to help avoid complications. In addition to Onodi cell configuration, other anatomic variants resulting in increased exposure of the optic nerve as well as dehiscence of the bony covering of the optic nerve canal are potential predisposing factors for catastrophic injury and should be noted on preoperative scans. One of these is anterior clinoid pneumatization (
Table 1.2).49 Regardless of whether the actual classification is used in routine clinical practice, awareness of these variations and potential exposure of the optic nerve by the otolaryngologist on preoperative scans is essential to help avoid complications. In addition to Onodi cell configuration, other anatomic variants resulting in increased exposure of the optic nerve as well as dehiscence of the bony covering of the optic nerve canal are potential predisposing factors for catastrophic injury and should be noted on preoperative scans. One of these is anterior clinoid pneumatization ( Fig. 1.16), which has been found to have an increased association with optic nerve dehiscence and considered an indicator of optic nerve vulnerability during endoscopic sinus surgery.6,49
Fig. 1.16), which has been found to have an increased association with optic nerve dehiscence and considered an indicator of optic nerve vulnerability during endoscopic sinus surgery.6,49
Fig. 1.18 (a,b) Examples of lateral pneumatization of the sphenoid sinus extending lateral to the foramen rotundum (arrows) and Vidian canals (small arrowheads). Coronal reformats from sinus CT scans of two different patients demonstrate prominent inferolateral sphenoid sinus pneumatization and variable extension of the Vidian canal within the sinus. (b) The left Vidian canal travels within a septation extending deep within the sinus.
Table 1.2 DeLano’s classification of the relationship of the optic nerve to the posterior paranasal sinuses49
Type | Description | Contact with posterior ethmoid air cells |
1 | Course adjacent to the sphenoid sinus without indentation of the sphenoid sinus wall | No |
2 | Course adjacent to the sphenoid sinus, causing indentation of the sphenoid sinus wall | No |
3 | Course through the sphenoid sinusa | No |
4 | Course immediately adjacent to the sphenoidal sinus and the posterior ethmoidal air cells | Yes |
a This may be defined as at least 50% of the nerve being surrounded by air/pneumatized sinus.6
The sphenoid sinus roof, the planum sphenoidale, is thin and vulnerable to perforation during surgery. The other sinus walls are of variable thickness, depending on the degree of pneumatization. As discussed earlier, when the sphenoid sinuses are well developed, many important neighboring structures can be identified by their indentation into the sinus cavity, including Vidian canal and the foramen rotundum (maxillary nerve [V2]), optic nerve, and the internal carotid artery, among others. In some cases, bony ridges or septations may be present on some of these structures ( Fig. 1.16). Areas with dehiscent walls are potentially susceptible to perforation during surgery. This is especially so with regard to the planum sphenoidale, the lateral sinus wall, and the medial roof of a lateral sinus recess into the greater sphenoid wing or pterygoid process. The latter area is also frequently the site of spontaneous CSF leak secondary to slow erosion resulting in an osteodural defect, as discussed earlier (
Fig. 1.16). Areas with dehiscent walls are potentially susceptible to perforation during surgery. This is especially so with regard to the planum sphenoidale, the lateral sinus wall, and the medial roof of a lateral sinus recess into the greater sphenoid wing or pterygoid process. The latter area is also frequently the site of spontaneous CSF leak secondary to slow erosion resulting in an osteodural defect, as discussed earlier ( Fig. 1.19).
Fig. 1.19).
The posterior ethmoid surface and the anterior face of the sphenoid sinus share a common wall that is divided by the perpendicular attachment of the superior turbinate.6 The ostium of the sinus lies in the upper portion of the intranasal surface and drains into the sphenoethmoidal recess, as discussed earlier. Because of its position and location, the normal drainage of the sphenoid sinus in the erect posture relies entirely on ciliary action. The sphenopalatine artery crosses the face of the sphenoid below the ostium. As such, during procedures aimed at enlarging the natural sphenoid sinus ostium, this artery may have to be cauterized. Air cells may be present within the posterosuperior part of the nasal septum and, when present, usually communicate with the sphenoid sinus. These can become inflamed like other sinus air cells and should be recognizable on CT and if necessary using MRI to distinguish from other pathology.
Arterial supply to the sphenoid sinus is derived from the posterior ethmoidal branches of the ophthalmic arteries (supplied by the internal carotid arteries) and the sphenopalatine branches of the maxillary artery (supplied by the external carotid arteries). Venous drainage is through the maxillary vein (and therefore there is a communication with the pterygoid plexus of veins) and the posterior ethmoidal veins into the superior ophthalmic veins. The lymphatics drain into the retropharyngeal lymph nodes. Innervation of the sphenoid sinus is through the posterior ethmoidal nerve, a branch of the nasociliary nerve (supplied by the ophthalmic [V1] division of trigeminal nerve) as well as the sphenopalatine branches (from the maxillary [V2] division of the trigeminal nerve) to the floor of the sinus.6
Paranasal sinus drainage pathways
Overview of paranasal sinus drainage pathways
Normal paranasal sinus drainage is through the coordinated ciliary action of its mucosal lining, propelling secretions toward the natural ostia. Given the location of the sinus ostia, in the erect position, drainage is largely accomplished by intact ciliary action. Successful drainage also requires intact/patent drainage pathways. As such, understanding the main drainage pathways and associated landmarks is key for the evaluation of sinus anatomy and these may best be considered as functional units. Obstruction at key sites within these functional units results in a predictable pattern of sinus obstruction.
Fig. 1.20 Coronal reformatted (a) and axial (b,c) images from a sinus CT demonstrate an example of obstruction of the ostiomeatal unit or complex (OMU) at the convergence of the drainage of the frontal, anterior ethmoid, and maxillary sinuses resulting in a predictable pattern with opacification of those sinuses.
The main drainage for the maxillary sinus is via the maxillary ostium into the infundibulum, hiatus semilunaris, and then the middle meatus ( Fig. 1.12). The frontal sinus drains through the frontoethmoidal recess (
Fig. 1.12). The frontal sinus drains through the frontoethmoidal recess ( Fig. 1.14) into the middle meatus, where it joins flow from ipsilateral maxillary sinus. The anterior ethmoid complex drains via the ethmoid bulla and hiatus semilunaris into the middle meatus. The posterior ethmoid complex and sphenoid sinus drain into sphenoethmoidal recess, then into superior meatus and subsequently nasopharynx (
Fig. 1.14) into the middle meatus, where it joins flow from ipsilateral maxillary sinus. The anterior ethmoid complex drains via the ethmoid bulla and hiatus semilunaris into the middle meatus. The posterior ethmoid complex and sphenoid sinus drain into sphenoethmoidal recess, then into superior meatus and subsequently nasopharynx ( Fig. 1.11). The inferior meatus receives drainage from nasolacrimal duct.
Fig. 1.11). The inferior meatus receives drainage from nasolacrimal duct.
Ostiomeatal unit
The ostiomeatal unit or complex (OMU) refers to a functional unit of structures draining the frontal, anterior ethmoid, and maxillary sinuses ( Fig. 1.12). It includes the middle meatus, the ethmoid bulla, the uncinate process, hiatus semilunaris, the infundibulum, and the superomedial maxillary sinus/maxillary sinus ostium.3 The hiatus semilunaris is the area between uncinate process and ethmoid bulla that receives drainage from anterior ethmoid air cells and maxillary sinus (via the infundibulum;
Fig. 1.12). It includes the middle meatus, the ethmoid bulla, the uncinate process, hiatus semilunaris, the infundibulum, and the superomedial maxillary sinus/maxillary sinus ostium.3 The hiatus semilunaris is the area between uncinate process and ethmoid bulla that receives drainage from anterior ethmoid air cells and maxillary sinus (via the infundibulum;  Fig. 1.12). Familiarity with this functional unit and pattern of drainage is important and obstruction of the unit can result in a predictable pattern of sinus opacification (
Fig. 1.12). Familiarity with this functional unit and pattern of drainage is important and obstruction of the unit can result in a predictable pattern of sinus opacification ( Fig. 1.20).
Fig. 1.20).
Based on the structures constituting and surrounding the OMU, different anatomic variants can be important for preoperative assessment and planning of procedures involving this drainage pathway. Haller cells, ethmoid bulla, and concha bullosa were discussed previously. Pneumatization of uncinate process or uncinate bulla represents an additional potential predisposing factor for impaired sinus ventilation. It is believed to be caused by extension of the agger nasi cell within the anterosuperior portion of the uncinate process.
When encountering an OMU pattern of obstruction, one important entity that should be recognized and distinguished is the atelectatic sinus or silent sinus syndrome.50 This occurs when the free edge of the uncinate process is rotated laterally or adherent to the orbital floor or inferior part of the lamina papyracea. This configuration, possibly combined with superimposed inflammation, results in occlusion of the infundibulum ( Fig. 1.21). This in turn is believed to result in negative pressure formation in the sinus that could in turn result in further rotation and retraction of the infundibulum, exacerbating and propagating the obstruction. The characteristic imaging findings are a retracted, adherent uncinate process with the absence of patent infundibulum, retracted maxillary sinus walls, and an enlarged ipsilateral middle meatus secondary to the retracted medial maxillary sinus wall (
Fig. 1.21). This in turn is believed to result in negative pressure formation in the sinus that could in turn result in further rotation and retraction of the infundibulum, exacerbating and propagating the obstruction. The characteristic imaging findings are a retracted, adherent uncinate process with the absence of patent infundibulum, retracted maxillary sinus walls, and an enlarged ipsilateral middle meatus secondary to the retracted medial maxillary sinus wall ( Fig. 1.21).50 Clinically, this can lead to painless spontaneous enophthalmos, hypoglobus, and facial deformities and asymmetries. When encountered, it is important to recognize this entity and not confuse with a developmentally hypoplastic maxillary sinus. For surgical planning, it is also important to note that in these cases the ipsilateral orbital floor can be low-lying, increasing the risk of inadvertent penetration of the orbit during surgery.6
Fig. 1.21).50 Clinically, this can lead to painless spontaneous enophthalmos, hypoglobus, and facial deformities and asymmetries. When encountered, it is important to recognize this entity and not confuse with a developmentally hypoplastic maxillary sinus. For surgical planning, it is also important to note that in these cases the ipsilateral orbital floor can be low-lying, increasing the risk of inadvertent penetration of the orbit during surgery.6
Fig. 1.21 Atelectatic maxillary sinus. Characteristic imaging findings are shown including a retracted adherent uncinate process with absence of patent infundibulum (arrows), retracted maxillary sinus walls, and an enlarged ipsilateral middle meatus (MM) secondary to a retracted medial maxillary sinus wall. MS, maxillary sinus; MT, middle turbinate.
Frontal recess
Each frontal sinus drains through an inferiorly located ostium into the frontal recess or frontal sinus drainage pathway ( Fig. 1.14). The frontal recess is a somewhat hourglass-shaped narrowing between the frontal sinus and the anterior middle meatus providing the pathway for drainage of the frontal sinus. Its relations are the lamina papyracea laterally, the middle turbinate medially, the posterosuperior wall of the agger nasi cell (if present) anteriorly, and the anterior wall of the ethmoid bulla posteriorly.6
Fig. 1.14). The frontal recess is a somewhat hourglass-shaped narrowing between the frontal sinus and the anterior middle meatus providing the pathway for drainage of the frontal sinus. Its relations are the lamina papyracea laterally, the middle turbinate medially, the posterosuperior wall of the agger nasi cell (if present) anteriorly, and the anterior wall of the ethmoid bulla posteriorly.6
The frontal sinus drainage pathway can be thought of as having a superior and an inferior compartment ( Fig. 1.14).26 The superior compartment is formed by the union of adjacent air spaces at the anteroinferior portion of the frontal bone and the anterosuperior portion of the ethmoid bone. The frontal ostium is at the upper border of the superior compartment. The superior compartment directly communicates with the inferior compartment.
Fig. 1.14).26 The superior compartment is formed by the union of adjacent air spaces at the anteroinferior portion of the frontal bone and the anterosuperior portion of the ethmoid bone. The frontal ostium is at the upper border of the superior compartment. The superior compartment directly communicates with the inferior compartment.
The anatomy of the inferior compartment of the frontal sinus drainage pathway can vary depending on the site of insertion of the uncinate process anteriorly.28 When the anterior portion of the uncinate process extends superiorly to attach to the skull base, this results in formation of the ethmoid infundibulum (constituting the inferior part of the frontal sinus drainage pathway) ( Fig. 1.14). The ethmoid infundibulum communicates with the middle meatus through the hiatus semilunaris. On the other hand, when the anterior portion of the uncinate process attaches to the lamina papyracea rather than the skull base, the inferior compartment of the frontal sinus drainage pathway becomes the middle meatus itself.
Fig. 1.14). The ethmoid infundibulum communicates with the middle meatus through the hiatus semilunaris. On the other hand, when the anterior portion of the uncinate process attaches to the lamina papyracea rather than the skull base, the inferior compartment of the frontal sinus drainage pathway becomes the middle meatus itself.
A number of anatomic variants and air cells may be present along the frontal sinus drainage pathway. Some are incidental and of no clinical significance, whereas others could be important for surgical planning and/or potentially result in narrowing of the pathway depending on their exact location and size. Agger nasi cells were discussed earlier. There are also other air cells in this area that could be important and should be recognized on preoperative scans, including four types of frontal cells.34 These are summarized in  Table 1.1.
Table 1.1.
Pterygopalatine Fossa
We will finish the section on the clinical anatomy of the anterior skull base and paranasal sinuses with a discussion of the PPF because of its important relationship to the skull base and paranasal sinuses. The PPF is an important site of anatomic convergence of multiple neural pathways. Familiarity with this area is critical, particularly for the evaluation of tumors where there is potential for perineural spread of tumor affecting treatment planning and management. The PPF is an elongated, vertically oriented, predominantly fat-filled region located lateral to the posterior aspect of the nasal cavity ( Fig. 1.22,
Fig. 1.22,  Fig. 1.23).51,52 The posterior wall of the maxillary sinus forms the anterior margin of the PPF. The posterior boundary of this space is composed of the fused pterygoid plates and the base of the sphenoid bone. The perpendicular plate of the palatine bone forms the majority of the medial margin of the PPF with the exception of the sphenopalatine foramen superiorly (
Fig. 1.23).51,52 The posterior wall of the maxillary sinus forms the anterior margin of the PPF. The posterior boundary of this space is composed of the fused pterygoid plates and the base of the sphenoid bone. The perpendicular plate of the palatine bone forms the majority of the medial margin of the PPF with the exception of the sphenopalatine foramen superiorly ( Fig. 1.22). The lateral boundary is the pterygomaxillary fissure, an elongated vertical communication with the infratemporal fossa (
Fig. 1.22). The lateral boundary is the pterygomaxillary fissure, an elongated vertical communication with the infratemporal fossa ( Fig. 1.22). The superior aspect is in continuity with the inferior orbital fissure (
Fig. 1.22). The superior aspect is in continuity with the inferior orbital fissure ( Fig. 1.22). Inferiorly, the PPF tapers into the greater palatine canal ultimately leading to the greater and lesser palatine foramina (
Fig. 1.22). Inferiorly, the PPF tapers into the greater palatine canal ultimately leading to the greater and lesser palatine foramina ( Fig. 1.22).
Fig. 1.22).
The neurovascular contents of PPF include the maxillary division of the trigeminal nerve, the pterygopalatine ganglion, and terminal branches of the internal maxillary artery. As alluded earlier, the PPF communicates with multiple adjacent spaces.3 Laterally, the pterygomaxillary fissure opens into the infratemporal fossa. Medially, the sphenopalatine foramen opens just posterior to the superior or middle meatus where the foramen is covered by mucosa. The sphenopalatine foramen transmits the sphenopalatine artery as well as the nasopalatine and superior nasal nerves from the PPF. Posteriorly, the foramen rotundum communicates with the middle cranial fossa, transmitting the maxillary (V2) branch of the trigeminal nerve. Inferior and medial to the level of the foramen rotundum, the Vidian (pterygoid) canal is located posteriorly and extends to the foramen lacerum. This canal transmits the Vidian nerve. Superolaterally, the inferior orbital fissure transmits the infraorbital nerve and artery. Inferiorly, the pterygopalatine canal leads to the greater and lesser palatine foramina and then the oral cavity.3 The greater palatine foramen transmits the greater palatine nerve and descending palatine vessels. The lesser palatine foramen transmits the lesser palatine nerves. Although commonly single, there can be two or rarely more than two lesser palatine foraminas.53
Fig. 1.22 Major imaging landmarks for the pterygopalatine fossa (PPF). (a,d) Axial images with (b) coronal and (c) sagittal (from the normal left side) reformats from a contrast-enhanced neck CT are shown from a patient with perineural spread of adenoid cystic carcinoma to the right PPF. On the reconstructions using soft-tissue windows (d), at a level slightly higher than in (a), note normal fat-filled left PPF containing normal vascular and neural structures. The right PPF, on the other hand, is infiltrated and mildly expanded (black arrow; d). Perineural spread of tumor can be subtle or not detectable on CT and is most accurately evaluated using MRI. GPC, greater palatine canal; IOF, inferior orbital fissure; MS, maxillary sinus; PPF, pterygopalatine fossa; PMF, pterygomaxillary fissure; SOF, superior orbital fissure; SPF, sphenopalatine foramen; SS, sphenoid sinus.
Fig. 1.23 Pterygopalatine fossa (PPF) and perineural spread of tumor (adenoid cystic carcinoma) on MRI. Axial (a) T1-weighted and (b) fat-suppressed postcontrast T1-weighted images from the same patient as in  Fig. 1.22 demonstrate perineural spread of tumor in the right PPF (arrow). Also note the appearance of the normal contralateral PPF.
Fig. 1.22 demonstrate perineural spread of tumor in the right PPF (arrow). Also note the appearance of the normal contralateral PPF.
1.4 Pathology of the Anterior Skull Base and Paranasal Sinuses
1.4.1 Tumors
Squamous Cell Carcinoma
Sinonasal cancers constitute a small percentage, approximately 5%, of all head and neck cancers with an estimated worldwide incidence of 1 per 100,000 annually.54 Among these, 50 to 80% are squamous cell carcinomas (SCCs). In the nasal cavity, males are affected more frequently than females and patients most commonly present between the ages of 55 and 65 years.55 Multiple occupational risk factors have been identified for SCC and sinonasal adenocarcinoma, including those resulting in exposure to nickel, wood furniture and leather production, chromium, mustard gas, isopropyl alcohol, formaldehyde, arsenic, and radium.55,56,57 Additional risk factors include a history of previous radiation therapy as well as immunosuppression and smoking.58,59 Thorotrast exposure is an established etiologic agent for maxillary sinus carcinoma, although practically no longer seen as the contrast agent has long been discontinued.55 SCC may develop concurrently within an inverted papilloma or subsequent to resection. Synchronous or metachronous SCCs of the same histologic type occur in 15%, most often outside of the head and neck. The most common tumor location is the maxillary antrum, followed by the nasal cavity and ethmoid sinuses. Much less commonly, the sphenoid and frontal sinuses are the sites of tumor origin.
Imaging characteristics
On CT, SCC of the sinonasal cavities has the appearance of a soft-tissue mass with variable contrast enhancement ( Fig. 1.24,
Fig. 1.24,  Fig. 1.25,
Fig. 1.25,  Fig. 1.26). Aggressive and relatively extensive osseous destruction is often present, while bone remodeling and/or sclerosis are less common (
Fig. 1.26). Aggressive and relatively extensive osseous destruction is often present, while bone remodeling and/or sclerosis are less common ( Fig. 1.24,
Fig. 1.24,  Fig. 1.25,
Fig. 1.25,  Fig. 1.26).55 Corresponding with osseous destruction, there may be alteration of fat attenuation or frank soft-tissue extension in adjacent regions such as the retromaxillary fat and the extraconal orbit. CT also directly delineates the cribriform plate, lateral lamella, fovea ethmoidalis, and orbital walls (
Fig. 1.26).55 Corresponding with osseous destruction, there may be alteration of fat attenuation or frank soft-tissue extension in adjacent regions such as the retromaxillary fat and the extraconal orbit. CT also directly delineates the cribriform plate, lateral lamella, fovea ethmoidalis, and orbital walls ( Fig. 1.27). It should be noted that the appearance of the normal cribriform plate can include small focal lucencies and these do not imply erosion secondary to tumor or invasion.60 In cases of early focal invasion, the difference can be subtle and requires careful evaluation. Intracranial transgression can be determined by identification of tumor density or intensity tissue extending through the defect to the intracranial aspect of the bone (
Fig. 1.27). It should be noted that the appearance of the normal cribriform plate can include small focal lucencies and these do not imply erosion secondary to tumor or invasion.60 In cases of early focal invasion, the difference can be subtle and requires careful evaluation. Intracranial transgression can be determined by identification of tumor density or intensity tissue extending through the defect to the intracranial aspect of the bone ( Fig. 1.28). In this regard, MRI is superior for tumor delineation and evaluation of intracranial extension and should be performed if there is ambiguity regarding skull base invasion and intracranial extension.
Fig. 1.28). In this regard, MRI is superior for tumor delineation and evaluation of intracranial extension and should be performed if there is ambiguity regarding skull base invasion and intracranial extension.
Perineural extension may be apparent as evidenced by effacement and infiltration of normal fat in key neurovascular conduits such as the PPF ( Fig. 1.23). In advanced cases, tubular soft-tissue structures may be visible extending along adjacent neural pathways (
Fig. 1.23). In advanced cases, tubular soft-tissue structures may be visible extending along adjacent neural pathways ( Fig. 1.29), sometimes with associated osseous destruction or remodeling at the sites of skull base foramina including the Vidian (pterygoid) canals, foramen rotundum, and foramen ovale. In advanced and longstanding cases, unilateral denervation changes such as muscular atrophy and fatty replacement in the territory of an involved motor nerve such as the mandibular division of the trigeminal nerve can be present (
Fig. 1.29), sometimes with associated osseous destruction or remodeling at the sites of skull base foramina including the Vidian (pterygoid) canals, foramen rotundum, and foramen ovale. In advanced and longstanding cases, unilateral denervation changes such as muscular atrophy and fatty replacement in the territory of an involved motor nerve such as the mandibular division of the trigeminal nerve can be present ( Fig. 1.29). Denervation changes should not be confused with direct tumor spread and invasion. Apart from differences in signal intensity depending on the stage of denervation, one clue for identifying denervation change is that while the signal in the muscles may be abnormal, their overall architecture and shape is preserved, unlike muscle invaded by tumor.
Fig. 1.29). Denervation changes should not be confused with direct tumor spread and invasion. Apart from differences in signal intensity depending on the stage of denervation, one clue for identifying denervation change is that while the signal in the muscles may be abnormal, their overall architecture and shape is preserved, unlike muscle invaded by tumor.