Key Points
- ▪
CT scanning is the dominant imaging test for the assessment of acute aortic diseases.
- ▪
Without ECG-gating, motion artifacts are still a problem for assessment of the ascending aorta and root; ECG-gating obviates such artifacts and provides superlative imaging detail for what was formerly a problem area for CT imaging.
- ▪
Knowledge of the range of aortic lesions and their complications is imperative.
CT scanning has emerged as the de facto test of choice for the identification of diseases of the aorta. As a result of its widespread and 24-hour availability, its suitability to evaluate critically ill patients (as long as they can be moved), and the appropriateness of its spatial and temporal resolution to the needs of most aortic pathologies, CT scanning has become the principal test for the evaluation of diseases of the aorta. Although CT scanning is the leading test to diagnose structural disease of the aorta, it is less able to diagnose the functional consequences of such disease. Often, therefore, CT scanning is combined with another modality to establish both the anatomic and functional disturbances of aortic disease. For example, although both CT scanning and echocardiography are strong tests to identify dissection of the aorta, most patients are managed with both, because CT identifies a far greater extent of the aorta than echocardiography does, but echocardiography is better for identifying cardiac complications (e.g., aortic insufficiency, cardiac tamponade and myocardial ischemia). Integrated imaging strategies remain the most clinically solid approach.
The only issues limiting the use of CT are the risks of radiation and of contrast-related renal insufficiency. To reduce the risks of radiation where they are most harmful, therefore, evaluation of aortic congenital malformations (such as coarctation) in very young patients is performed by magnetic resonance imaging or angiography (MRI/MRA).
Sixteen-slice CCT was already such a powerful modality for the diagnosis of the structural aspects of aortic disease that the improvements that occurred as 40- and 64-slice CCT were developed to assess cardiac (coronary) disease only enhanced CCT’s dominance. Imaging techniques that struggle with coronary artery imaging are generally more successful with aortic imaging because the aorta is almost a logarithm greater in size and is subject to less motion than the coronary arteries.
Normal thoracic aortic diameters overall and by gender are presented in Table 26-1 .
| OVERALL ( n = 103), MEAN (SD) | WOMEN ( n = 44), MEAN (SD) | MEN ( n = 59), MEAN (SD) | P value | |
|---|---|---|---|---|
| Aortic Root | ||||
| Diameter, short-axis ED (cm) | 3.1 (0.3) | 2.9 (0.2) | 3.2 (0.3) | <.001 |
| Area, short-axis ED (cm 2 ) | 7.9 (1.6) | 6.9 (1.4) | 8.5 (1.7) | <.001 |
| Ascending Aorta | ||||
| Diameter, short-axis ED (cm) | 2.8 (0.4) | 2.8 (0.4) | 2.8 (0.3) | .61 |
| Diameter, short-axis ES (cm) | 3.0 (0.3) | 2.9 (0.4) | 3.0 (0.3) | .40 |
| Diameter, axial ES (cm) | 3.0 (0.3) | 3.0 (0.4) | 3.1 (0.3) | .46 |
| Descending Thoracic Aorta | ||||
| Diameter, short-axis ED (cm) | 2.1 (0.2) | 2.0 (0.2) | 2.2 (0.2) | .005 |
| Diameter, short-axis ES (cm) | 2.2 (0.2) | 2.2 (0.2) | 2.3 (0.2) | .011 |
| Diameter, axial ES (cm) | 2.3 (0.2) | 2.1 (0.1) | 2.3 (0.3) | <.001 |
CT Artifacts and Aortic Diseases
Although CT is the principal diagnostic test for diseases of the aorta, CT artifacts may confound assessment of aortic diseases. The older the CT scanner, the greater the number of artifacts (at least half of CT studies on scanners from 1999 or earlier have significant artifacts over the ascending aorta).
Potentially problematic artifacts include:
- □
Streak artifacts off the brachiocephalic vein (from contrast), confounding assessment of the aortic arch behind it
- □
Streak artifacts off the superior vena cava (from contrast or pacer wires), confounding assessment of the ascending aorta beside it
- □
Streak artifacts off the spine, confounding assessment of the descending aorta beside it
- □
Older scanners (slow, or without cardiac gating) may generate “pulsation” artifacts of superimposed images of the aorta in different positions due to translation from cardiac motion, conferring the impression of an intimal flap. There is almost 1 cm of translational motion of the ascending aorta through the cardiac cycle.
- □
Motion artifacts
- □
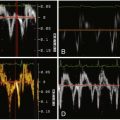
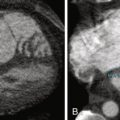
See Figure 26-1 .

Figure 26-1
The systolic motion (translation and pulsation) of the aorta on non–ECG-gated imaging may render a superimposition image artifact to suggest an intimal flap.
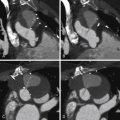
Atherosclerotic Calcification
Atherosclerotic plaques in the aorta are extremely common. The two sites most often involved are the abdominal aorta and the distal floor of the arch. Although there is general correlation between the extent of aortic and coronary atherosclerosis, many patients will have small flecks of aortic calcification without having significant coronary disease.
Intimal calcification is a marker of intimal position. “Intimal displacement” (into the lumen) is a convenient sign of mural thickening due to either occurrence of a false lumen within the wall of the aorta or development of intramural hematoma. If there is thickening over (i.e., on the luminal side of) intimal calcification, it usually is from thrombus, particularly if the aorta is dilated at that site.
For images of calcification of the aorta, see Figures 26-2 and 26-3 .


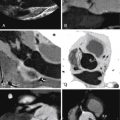
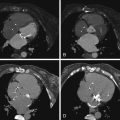
Aortic Dissection
CT is the principal modality used to diagnose acute aortic dissection (AAD). CCT has emerged as the initial diagnostic modality to identify or exclude AAD by virtue of:
- □
Imaging both the thoracic and abdominal aorta (vs. echocardiography), which has far more limited field-of-view
- □
Its speed (vs. MRI)
- □
Its very high degree of accuracy (vs. angiography)
- □
Its ability to image the majority of side branches (vs. echocardiography)
- □
Its ability to image important variants of dissection such as intramural hematoma (vs. angiography)
- □
The fact that most referring physicians are comfortably familiar with it
Studies comparing CT to other modalities were numerous 12 to 15 years ago; however, few studies have been done involving the current generation of CT equipment. Studies from a decade ago cannot depict the relative accuracy of any test that has developed as much as CCT has in the past 5 years. To further complicate comparisons, TEE and MRI also have evolved over the last decade.
Findings of aortic dissection that CCT is able to image include:
- □
Intimal flap
- □
False lumen. The false lumen generally arises off the outer curvature of the aorta (as the tear is usually located on the outside curvature).
- □
Thrombosis within the false lumen (suggests/consistent with chronicity or healing)
- □
Entry tear. The two most common sites of entry tears are 2 cm above the aortic valve and 1 cm distal to the left subclavian artery.
- □
Exit (re-entry) tear
- □
Pleural effusion
- □
Pericardial effusion
- □
Mediastinal hematoma
- □
Branch vessel involvement. The finding that a branch vessel arises from the false lumen does not establish that its vascular bed is underperfused, only that it is jeopardized. One of the few signs of vascular bed hypoperfusion that is clinically supported is that of pallor of the kidneys during a contrast-enhanced scan.
- □
Coronary artery involvement, especially if ECG gating is employed
The diagnosis of a dissection is made when an intimal flap is identified. Lack of thrombosis within the false lumen supports acuity of the dissection, while thrombosis supports chronicity.
Potential false positives that may occur include streak artifact from residual contrast in the SVC across the ascending aorta appearing as linear artifact masquerading as an intimal flap. The linearity of the line artifact across the ascending aorta, the presence of multiple lines, the presence of nearby bright lines, and the traversing of tissue planes by the lines indicate that these are artifact. Intimal flaps are not straight in their entirety.
CCT is the most commonly used modality for following aortic diameter in patients with aortic dissection in the chronic phase and is able to identify progressive enlargement as well as persistence of flow in the false lumen. Aortic diameter of type B (distal) dissection cases increases in 37% of cases with a thrombosed false lumen and in 90% of cases with a persistent false lumen. Growth rate has been noted to be 4 ± 7 mm/year in the ascending aorta, 2 ± 9 mm/year in the arch, and 1 ± 6 mm/year in the descending aorta.
For images of acute aortic dissection, see Figures 26-4 through 26-13 and










Intramural Hematoma
An important variant of aortic dissection is acute intramural hematoma (IMH; Figs. 26-14 through 26-17 ). Approximately 10% of suspected dissection cases are IMH. Merely ruling out dissection does not achieve the diagnosis of this equally important, underrecognized pathology. There are fewer data delineating the natural history, prognostic features, and outcomes of management strategies on IMH than on AAD. To summarize what is known:
- □
Outcomes parallel those of AAD.
- □
Description of the anatomic site of involvement using “Type A/Ascending” and “Type B/Descending/Non-Ascending”) as per AAD classification is suitable. The only location of IMH and AAD that does not lend itself to description by this classification is that of isolated arch involvement.
- □
Both non–contrast- and contrast-enhanced views are useful to depict the mural thickening of IMH.
- □
The appearance usually is of a crescentic thickening of the wall. Occasionally the mural thickening is circumferential (“annular”).
- □
The longitudinal extent of involvement is generally much less than that of AAD (8–12 cm only). Unlike AAD, most IMH do not involve the entire aorta.
- □
Risk appears related to:
- •
Proximal location
- •
Leakage
- •
Greater anatomic extent of involvement
- •
Association with atherosclerotic penetrating ulcer
- •
- □
There is no intimal tear or connection of the false lumen to the true lumen.
- □
The differential is of aortic dissection with a thrombosed false lumen.
- □
About 5% to 10% of cases will, in time, become frank dissection with an intimal tear connecting the true and false lumens.
Management is undertaken according to the paradigm of AAD management:
- □
Surgery for proximal involvement
- □
Medical management for uncomplicated distal involvement
- □




CT is highly accurate (100%) at identifying IMH.
The aspect of surgery that is theoretically less well suited to address IMH pathology is aortic root replacement for AAD, which has a high chance of eliminating the entry tear, thus depressurizing the false lumen distal to the root replacement and facilitating its thrombosis. In IMH, there is no entry tear to eliminate. Root replacement in the context of IMH provides protection to the aortic valve and coronary artery ostia, as well as to the pericardial space.
Identification of a penetrating ulcer responsible for IMH establishes a significantly worse prognosis. Sustained or recurrent pain, increasing left pleural effusion, increasing maximal aortic diameter, and increasing penetrating ulcer depth reliably predict progression and risk. Development of an “ulcer-like” projection of the ascending aorta or arch is a sign of progression to overt dissection.
Ulcers, Penetrating Ulcers, and Overlying Aortic Thrombus of the Aorta
The least common acute aortic syndrome is penetrating ulcer of the aorta. Atherosclerotic plaques in the aorta, as elsewhere (e.g., carotid and coronary trees), may ulcerate. Atherosclerotic plaques may eventually extend to variable depths (i.e., penetrate) within the wall of the aorta. The deeper the extent into the wall, the greater the chance of disruption of the integrity of the wall.
Weakening of the plaque that allows for communication of the lumen with deeper sections of the wall may result in propagation of blood into the wall and formation of an intramural hematoma within the wall. It also may result in leakage of blood out of the aorta (with or without an intramural hematoma), and, rarely, rupture of the aorta may occur.
CT is able to image aortic ulcers and penetrating ulcers. The challenge for all imaging modalities, including CT, is to distinguish between the two. Ulcers are common; penetrating ulcers are not. Ulcers will not lead to disruption of the aorta, whereas penetrating ulcers may do so. Ideally, imaging modalities would have sufficient resolution to depict the layers of the wall of the aorta distinct from the plaque itself. However, this is not always the case. Depiction of a deep “button-shaped” ulcer is usually convincing evidence of penetration.
Thrombi of variable size usually form on atheromatous plaques and may embolize. They commonly coexist.
Thrombosis and thromboembolism are other complications of atherosclerotic plaques of the aorta. Thrombosis is evident only on contrast-enhanced views as contrast void. A prominent plaque usually underlies the thrombus.
For images of atherosclerosis of the aorta, see Figures 26-18 and 26-19 .


For images of atherothrombosis of the aorta, see Figures 26-20 and 26-21 .


Occlusion of the Aorta
Occlusion of the aorta may be acute, resulting from either LV apical or left atrial thromboembolism, from local thrombosis often seen in low flow-shock states, from intra-aortic balloon counterpulsation, or, rarely, from more proximal aortic embolization of thrombus. Occlusion will only be apparent on contrast enhancement. Chronic occlusion usually is associated with extensive collaterals, which depend on the level of occlusion and may be caused by atherosclerosis or aortitis.
For images of occlusion of the aorta, see Figure 26-22 .

Middle Aortic Syndrome
“Middle aortic syndrome” is isolated abdominal level stenosis, which has many clinical similarities with coarctation of the thoracic aorta. It may be caused by aortitis.
For images of the middle aortic syndrome, see Figure 26-23 .

Aortic Aneurysm
CT scanning is extremely well suited to diagnosing and assessing aneurysmal disease of the aorta: thoracic, abdominal and thoracoabdominal ( Figs. 26-24 through 26-30 ). CT scanning is able to:
- □
Identify aneurysms
- □
Establish anatomic extent
- □
Establish (accurately) aneurysm diameter
- □
Distinguish aneurysms from dissection and false aneurysm
- □
Identify mural thrombus within aneurysms
- □
Identify leakage
- □
Identify the relation of the aneurysm to branch vessels
- □
Establish interventional suitability and planning
- □
Establish the relation of aneurysms of the aortic root and sinuses to coronary arteries