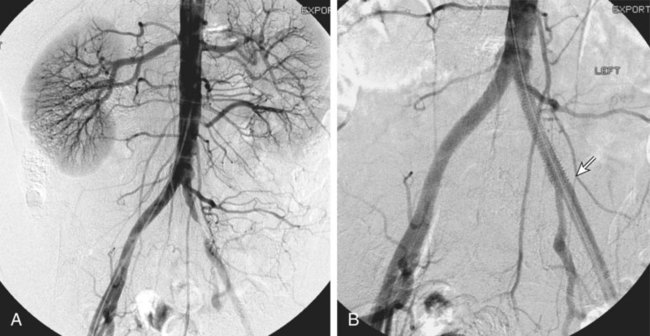
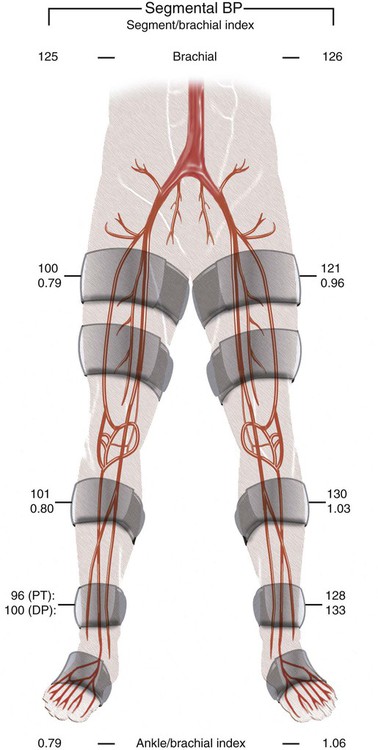
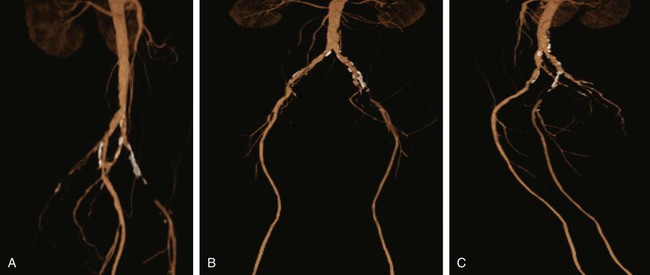
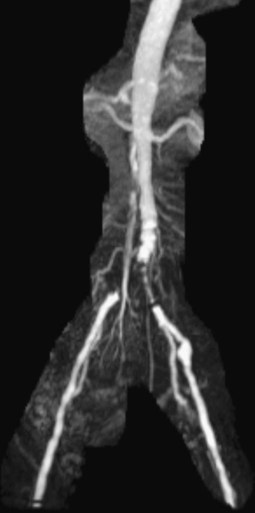
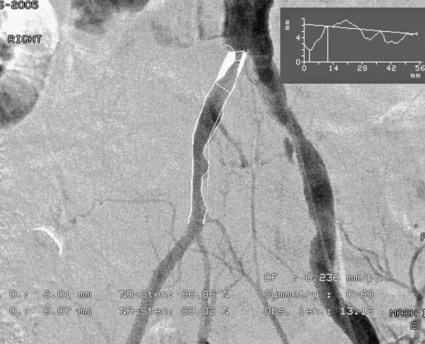
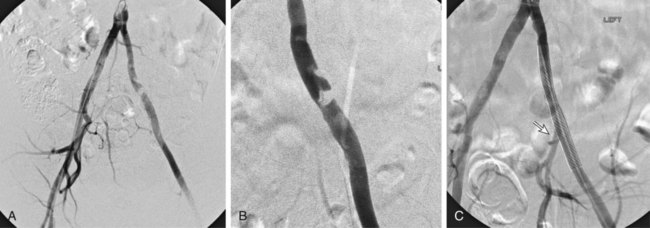
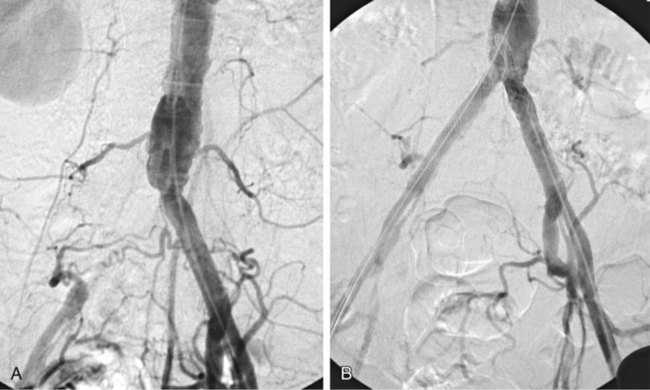
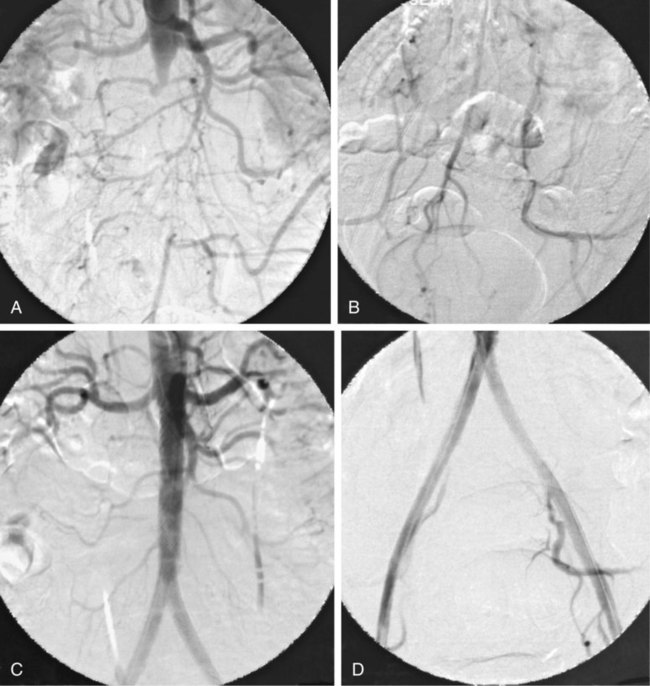
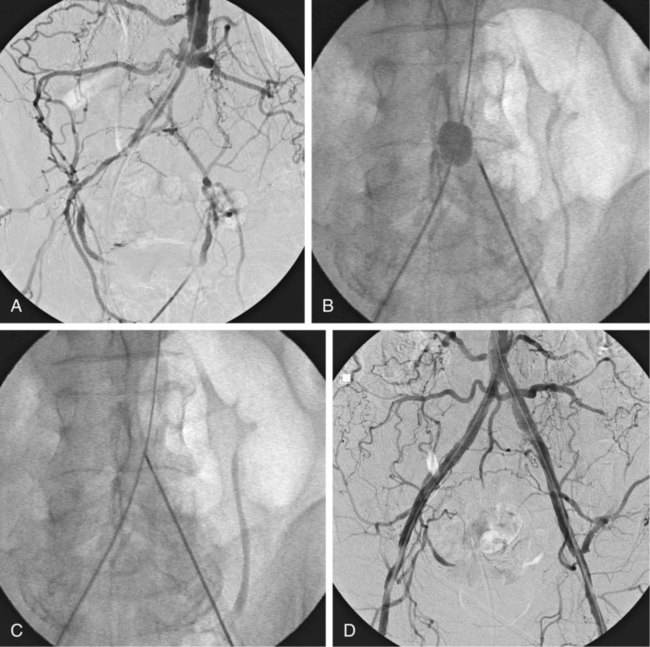
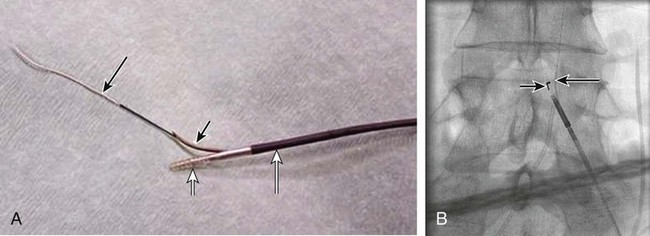
Chapter 35 Abdel Aziz A. Jaffan and Timothy P. Murphy Peripheral arterial disease (PAD) is a common medical condition that is underdiagnosed, undertreated, poorly understood, and much more common than previously thought.1 According to the Framingham (Massachusetts) Heart Study results, annual incidence of symptomatic PAD is 26 : 10,000 in men and 12 : 10,000 in women; PAD is at least as frequent as angina in the U.S. population.2 Historical prevalence data vary with the method of detection. Questionnaires designed to elicit symptoms, such as the Rose Questionnaire, typically underestimate the prevalence of PAD. Relying on the presence of intermittent claudication (IC) alone to make a diagnosis or screen for PAD would miss the majority of patients. In community cohort studies like the Rotterdam Study and the Edinburgh Artery Study, classic symptoms of IC occurred only in a minority (6%-9%) of patients.1,3–5 Population studies using noninvasive tests such as the ankle-brachial index (ABI) are more likely to yield a truer estimation because asymptomatic occurrences would also be included. ABI is currently accepted as a diagnostic reference standard for PAD, with a high sensitivity and specificity. It is noninvasive, inexpensive, and readily available.6 Criqui et al., using segmental blood pressures, flow velocity on Doppler ultrasound, postocclusive reactive hyperemia, and pulse return half-times, reported a prevalence of large vessel PAD of 11.7% in a population with an average age of 66 years.7 The prevalence of PAD is also related to age, as shown by Kannel in the Framingham Study, who found 2.5%, 8.3%, and 18.8% of Americans aged 60 years or younger, 61 to 70 years, and older than 70 years, respectively, to have PAD.8 In 2005, the Centers for Disease Control and Prevention (CDC) estimated that PAD affected 5% of the U.S. population older than 40 years of age.9 Higher prevalence is anticipated in the future as the average age of the U.S. population rises. Among those with advanced age (>70 years), history of smoking, and presence of diabetes mellitus, prevalence of PAD can be as high as 50%.3 In general, prevalence of PAD is estimated at 3% in middle-aged patients when noninvasive testing is used, increasing to 20% in patients older than 70. The aortoiliac territory is affected in one third of theses cases.10 In 1964, radiologists Dotter and Judkins published a seminal paper describing the first cases of percutaneous transluminal revascularization.11 What followed was a revolutionary change in management of PAD, best exemplified by aortoiliac interventions. The first iliac artery angioplasty was done using a “caged” compliant Fogarty balloon in 1965 by Dr. Charles Dotter12,13 shortly after he devised the method of serial dilation of the femoropopliteal arteries.11 Consistently good outcomes were achieved with conventional angioplasty and stent techniques in the aorta and iliac arteries. In an analysis of treatment patterns of aortoiliac occlusive disease in the United States, Upchurch et al. reported more than an eight fold increase in angioplasty and stenting, 15.5% decrease in aortobifemoral bypass surgery, and 34% increase in total number of interventions from 1996 to 2000.14 Endovascular therapy is generally accepted as the primary mode of revascularization therapy for most patients with aortoiliac disease. Although thigh and/or buttock claudication can help localize PAD symptoms to the proximal segment, the most common symptom of aortoiliac insufficiency is calf claudication. Patients can present with critical limb ischemia and tissue loss, but usually these patients have multisegmental disease involving infrainguinal arteries in addition to the aortoiliac segment. Erectile dysfunction in males can also be seen. Rarely, ulcerated plaques can result in cholesterol or thrombotic embolization and result in blue toe syndrome.1,3 Claudication is managed conservatively or by percutaneous or surgical revascularization. A previous report of relatively benign natural history of claudication,2 which may have fostered a conservative attitude, is contradicted by more recent studies.15,16 Of those who present with typical IC, after 5 years, 70% to 80% will have stable claudication, 10% to 20% will have worsening claudication, and 5% to 10% will develop critical limb ischemia. Less than 2% will undergo a major amputation.17 In a review of over 2307 patients in six studies, 30% of patients with IC eventually required surgery for critical limb ischemia, and 6% required amputations.16 Exercise programs have shown improvements in quality of life and walking distance, but benefits gained in an unsupervised setting are poor.18 Surgery, in combination with an exercise program, has proven superior to both exercise or surgery alone, albeit with higher complications.19 Despite excellent long-term outcome, surgical revascularization is associated with up to 4.6% perioperative mortality and a 13.1% major early complication rate.20 With the significant improvement in endovascular techniques and materials, endovascular therapy can be now considered the initial safe and effective therapeutic option for aorto-occlusive disease, with a high rate of technical success and excellent long-term patency rates.21 The TransAtlantic Inter-Society Consensus (TASC) document classified aortoiliac disease by angiographic appearance and pattern (see Chap. 25, Fig. 25-9). Initially published in 2000 and intended to provide recommendations regarding percutaneous vs. surgical revascularization based on severity of anatomic obstruction, it has been revised twice, each time broadening the indications for percutaneous revascularization. Briefly, TASC categorizes lesions of increasing severity, with TASC A lesions being focal and singular, and advancing categories describing longer, multiple, or occlusive lesions, through category D. It is the authors’ opinion that TASC is useful as a descriptive description of anatomy but does not impact clinical decision making in the aortoiliac segment because nearly all disease types are amenable to percutaneous revascularization, and except in cases of total aortoiliac occlusion from the renal arteries to the inguinal ligaments, where the patient has a good surgical risk profile, surgery should be reserved for those who fail percutaneous revascularization.22–26 Contraindications to arteriography generally apply also to aortoiliac interventions. Patients with uncorrectable coagulopathy, inability to lie supine, severe non–dialysis dependent renal insufficiency, or current systemic infections have relative contraindications to elective percutaneous revascularization, and alternate management should be considered until the risk profile is more suitable. Limited procedures can be performed with alternative contrast agents (namely, carbon dioxide) in patients with renal insufficiency, depending on the clinical situation. No absolute contraindications exist for endovascular treatment of aortoiliac disease per se. Relative contraindications are mainly anatomic and include juxtarenal aortic occlusion, circumferential heavy calcification (>1 mm in thickness), hypoplastic aortic syndrome, and juxtaposition to aneurysmal disease.27 A standard room for angiography equipped with single projection fluoroscopy, movable table, and power contrast injection pump are necessary. Most modern rooms for angiography have digital subtraction capability with features such as live road mapping and bolus-chasing capability. A sterile table should contain heparinized flush saline, hand-injectable contrast media, room for catheters and wires, lidocaine for dermal anesthesia, and a sharps container. The diagnostic portion of the examination can be performed using a multipurpose flush catheter. Femoral access can be challenging in patients with aortoiliac disease. Although most attempts at access can be attained using landmarks, ultrasound guidance may be useful. Alternatives to femoral access are brachial or even radial access.28 Continuous patient monitoring is essential and requires cooperation among the patient, nurse, technologist, and interventionalist. A cardiac monitor, pulse oximeter, and automated sphygmomanometer are mandatory. Emergent patient resuscitation equipment including a code cart should be readily available, and all providers should be Advanced Cardiac Life Support (ACLS) certified. Working familiarity with conscious sedation medications including reversal agents is required. Many types of stents are now available for use in the aortoiliac system. Stents can vary in their metallic composition, construction, radiopacity, trackability, flexibility, radial force, hoop strength, and foreshortening. For aortoiliac purposes, the vast majority of available stents can be divided into self-expanding types such as the Wallstent RP (Boston Scientific Corp., Natick, Mass.) or balloon-expandable types such as the Palmaz Genesis (Cordis Corp., Miami Lakes, Fla.). Self-expanding stents tend to have greater flexibility, trackability, and vessel wall apposition and are more likely to be crush resistant. Most balloon-expandable stents are made of stainless steel and possess greater hoop strength and radial force. The major advantage of balloon-mounted stents is the greater ability for precise placement—required, for example, for common iliac origin lesions or lesions extending to the iliac artery bifurcation (Fig. 35-1). Covered stents are increasingly used primarily in aortoiliac intervention, but definitely needed in case of vascular perforation; they can be self-expanding (e.g., Viahbahn [Gore Medical, Flagstaff, Ariz.]) or balloon-expandable (e.g., ICast [Atrium Medical, Hudson, N.H.]). With diffusion of technology, however, distinctions between stents are now becoming blurred. As a result, the authors stock a tailored list of stents, acquired over time with personal experience. Vascular sheaths, preferably with a radiopaque tip (e.g., Brite Tip [Cordis, Bridgewater, N.J.]), sizes between 5F and 10F and lengths up to 90 cm may be necessary to deliver appropriate-diameter balloons and stents, depending on the route of access. For chronic long-segment occlusions, subintimal recanalization is increasingly performed, and in many times is unavoidable. Once in the subintimal tract, reentry into the true lumen can pose a challenge. Reentry can be achieved using low-technology off-the-shelf devices like the back end of guidewires, stainless steel stiffening cannulas in combination with needle-tip guidewires,29 or with dedicated chronic total occlusion (CTO) reentry catheters (discussed later) like the Outback catheter (Cordis) and the Pioneer catheter (Medtronic, Minneapolis, Minn.), shown to increase technical success of crossing complete arterial occlusions. A reentry catheter should be available in case needed. Patients should be evaluated by the interventionalist before any procedure. A directed history and physical examination should be performed to determine the presence and severity of PAD. Physical examination may reveal diminished or absent femoral artery pulses and decreased ABI. Other vascular systems should be examined (e.g., for carotid artery bruit or abdominal aortic aneurysm (AAA)). At the time of consultation, all available previous test results should be reviewed, including pulse volume recording (PVR), segmental pressures (Fig. 35-2), computed tomographic and magnetic resonance angiography (CTA, MRA), and records of prior interventions and surgeries. Necessary tests should be ordered. Pertinent laboratory values include platelet count, prothrombin time, and serum creatinine and blood urea nitrogen (BUN) levels. Presence of severe renal insufficiency may preclude use of iodinated contrast agents. There is a lack of consensus regarding appropriate contrast nephropathy prophylaxis, but most authors recommend (1) hydration with 0.9% saline at 1 mL/kg/h for 24 hours beginning at 2 to 12 hours before the procedure and (2) use of the least amount possible of low-osmolar iodinated contrast agent.30 Use of N-acetylcysteine and sodium bicarbonate infusion may be helpful, but results of clinical trials have been equivocal or limited. If a non–life threatening contrast allergy (e.g., urticaria) is present, a prophylaxis regimen such as 32 mg of oral methylprednisolone 12 and 2 hours before contrast agent administration has been recommended. A history of life-threatening allergic reaction (e.g., cardiopulmonary collapse, laryngeal edema, bronchospasm) should prompt an investigation of an alternative to contrast (e.g., carbon dioxide possibly supplemented with intraarterial (IA) pressure measurements during intervention). Insulin-dependent diabetics should take half their dose of insulin the morning of their procedure. Warfarin compounds and metformin should be held when appropriate. An assessment of cardiovascular risk in conjunction with their cardiologist should be done, and if anticoagulation cannot be stopped, they may need to be converted to subcutaneous low-molecular-weight heparin while the warfarin is held. It is not routine to evaluate patients for myocardial ischemia before percutaneous interventions. All patients with PAD also should be assumed to have significant coronary artery disease,31 but percutaneous revascularization is not contraindicated for such patients. With the advances and widespread availability of noninvasive imaging modalities, performing catheter arteriography for a purely diagnostic purpose is now rarely indicated. For aortoiliac evaluation, duplex arterial mapping, CTA, and MRA can provide very accurate anatomic details in terms of location and extent of disease and eligibility for endovascular intervention, without the need for the relatively invasive catheter angiography, its substantial costs, and small risk of complications.32 Duplex arterial mapping can provide a safe and inexpensive means of assessing the location and extent of hemodynamically significant stenosis in the aortoiliac territory. Limitations to this technique, including the presence of significant arterial calcifications, overlying bowel gas, large body habitus, and dependence on highly trained vascular technologists, made it fall out of favor as a primary tool for preprocedure noninvasive imaging.27 Developed shortly after the introduction of spiral (helical) computed tomography, CTA made it possible to cover body regions so rapidly, that transient enhancement of the vascular system following IV contrast injection would be captured in one scan (Fig. 35-3).33 With the introduction of multidetector (MD) row technology, spatial and temporal resolutions improved significantly, and CTA became an easy-to-perform standard technique for vascular imaging, taking over most diagnostic vascular procedures. Compared to intraarterial digital subtraction angiography (IA-DSA), CTA is noninvasive, permits three-dimensional (3D) visualization of vessels from any angle, has lower cost and shorter examination times, lowers overall effective radiation dose, and lowers the volume of intravascular contrast.34 Sensitivity and specificity of MD-CTA compared to conventional IA-DSA are reported to be between 93% and 100%. In a meta-analysis including 436 patients and 9541 arterial segments, Heijenbrok-Kal et al. found that MD-CTA has a sensitivity of 92% and a specificity of 93% for detection of hemodynamically significant (>50%) stenosis in patients with claudication or critical limb ischemia, with a diagnostic performance almost as good as IA-DSA.32 CTA is an accurate tool for assessment of all treatment-relevant morphologic information of PAD: gradation, length, and number of stenoses.35 Various 3D postprocessing techniques now available, such as maximum intensity projection (MIP), volume rendering (VR), and multiplanar reformatting (MPR), can improve diagnostic performance. Oto et al. found that curved MPR resulted in higher sensitivity (97% vs. 89%) and specificity (100% vs. 96%) than use of transverse images.36 A major drawback of MD-CTA is hampered vessel assessment due to arterial wall calcifications, which may cause overestimation of stenosis.33 Detection of calcification, however, has significant implications for treatment planning. Circumferential calcifications thicker than 1 mm are considered a relative contraindication to aortoiliac angioplasty and stenting because of the potential risk of fracture and rupture. A covered stent should be considered in this case to minimize the impact of a clinically significant arterial perforation.27 MRA is a noninvasive technique that does not employ ionizing radiation or iodinated contrast material (Fig. 35-4). Very fast 3D sequences in contrast-enhanced MRA (CE-MRA) allow study of the arteries from the abdominal aorta to the feet with a single injection of contrast material. With CE-MRA, the limitations of the older non-contrast MRA (NC-MRA) techniques (e.g., time of flight [TOF], phase contrast), such as long acquisition time and flow and movement artifacts, have been overcome.37 NC-MRA relies on inflow phenomena prone to saturation effects, which can lead to overestimation of the degree of stenosis in areas where the vessels run in a more horizontal course, such as the pelvis.38 CE-MRA is not susceptible to in-plane saturation. Other advantages include reduced imaging acquisition time and higher spatial resolution. In a multicenter controlled trial comparing NC-MRA, CE-MRA, and IA-DSA in 407 patients referred to investigate PAD in the aortoiliac region, Bui et al.38 showed that CE-MRA was superior to and more consistent than NC-MRA for detecting hemodynamically significant stenosis in patients with a suspected or proven PAD, and compared favorably with IA-DSA as a reference standard. A fundamental limitation to this modality is lack of visualization of vascular calcifications, important for surgical planning. Venous contamination is another limitation to this technique.37,38 Catheter arteriography is rarely performed nowadays for a purely diagnostic purpose, except in rare cases where noninvasive imaging is inconclusive. In patients undergoing catheter arteriography, a treatment plan has already been formulated and a decision for an endovascular therapy made. Common femoral artery (CFA) access is most desirable. Prior to the advent of CTA and MRA, puncture of the CFA was performed on the side with the better palpable pulse, and angiogram was performed to assess disease and decide whether a lesion should be treated from an ipsilateral or contralateral approach. Nowadays, preprocedural CTA/MRA should have determined the puncture site with the safest and most direct route to the target lesion. In general, common iliac artery (CIA) lesions are best treated ipsilaterally, and external iliac artery (EIA) lesions best approached from the contralateral access.39 If the lesion is close to the aortic bifurcation, bilateral CFA access for a kissing technique may be required; however, if the plaque is limited to the proximal CIA and does not involve stenosis of the distal aorta, our practice is to not deploy stents bilaterally but rather to use a balloon-expandable stent placed accurately to cover only the lesion in the CIA. If a femoral pulse is absent on the puncture side, fluoroscopically guided femoral access can be done using palpation in individuals whose body habitus permits because atherosclerotic arteries are often hard and can be palpated even if pulseless. If that is not the case, calcification is often present and permits access using fluoroscopy. Of course, ultrasound guidance can be used to gain access if these methods fail, and it is highly successful. Presence of calcifications in the wall of the vessel and the lateral location of the artery in the groin will help differentiate the CFA from the common femoral vein by ultrasound. Alternatively, brachial access can be obtained, especially in patients with complete aortoiliac occlusion. Once access is gained, a short 5F sheath is placed and set to a flush. A diagnostic arteriogram of the abdominal aorta and pelvis is obtained via a multisidehole flush catheter. Oblique projections may be helpful in the pelvis. Greater than 50% reduction of diameter is generally considered to represent hemodynamic significance. Commercially available software can be used to facilitate accurate measurements (Fig. 35-5). However, IA pressure measurements above and below the lesion are preferred because pressure gradients have been shown to be more accurate in detecting hemodynamically significant stenosis.40 Pressure gradients can be obtained simultaneously with single or double access, with the former requiring a 2F size difference between the coaxial catheter and the sheath. Pull-back measurement is less favored because blood pressure varies over time, and nonsimultaneous measurements may artifactually introduce a substantial difference (10-15 mmHg systolic) where none really exists, especially after pharmacologic augmentation. A systolic difference of 10 to 15 mmHg or a mean difference of 5 mmHg is considered significant. To mimic the increased flow situation that exists with exercise, patients with IC may require pharmacologic augmentation (e.g., IA nitroglycerin administered to the affected extremity) to unmask a hemodynamic significance when a lesion does not yield a pressure difference at rest. With pharmacologic augmentation, a difference of 20 mmHg systolic or 10 mmHg mean is necessary to be a significant gradient.40 For patients with critical limb ischemia, many clinicians use a gradient of 5 mmHg mean to indicate the need for revascularization.40,41 Pressure measurements are not routinely done for occlusions because it is self-evident that arterial occlusions are hemodynamically important. In the presence of occlusion, delayed images or ipsilateral access below the occlusion may be required to determine the exact length of the occluded vessel segment and the appropriateness of the artery to receive the distal portion of the stent. For example, if the occlusion extends below the inguinal ligament, an endovascular approach may not be beneficial. Usually, systemic anticoagulation is accomplished with intravenous (IV) heparin as soon as the determination for endovascular treatment course is made, but this is not universal, and simple procedures can be safely accomplished without anticoagulation if they can be done within a few minutes. Once the stenosis is traversed with a guidewire, a stent is properly positioned to cover the entire lesion and deployed. Negotiation of complex stenoses and occlusions can be challenging but facilitated with directable catheters and hydrophilic guidewires. Chronic occlusions are addressed later. Most patients will experience some level of discomfort due to adventitial stretching during inflation of the angioplasty balloon. Typically, 6 to 8 atmospheres of pressure is applied. Although arterial perforation can occur at lower pressures,42 the patient’s discomfort level should guide the degree of inflation. In cases of severe pain, aggressive dilation should be avoided. An appropriate level of conscious sedation is crucial to obtain real-time patient feedback. Self-expanding stents often require dilation to desired diameter with an angioplasty balloon and will usually recoil about 1 mm from the inflation diameter after the balloon is deflated. When placing a stent in the aortoiliac system, three areas merit special consideration. Stent extension below the inguinal ligament should be avoided, usually because it can interfere with future femoral endarterectomy or planned femoral distal bypass surgery. In circumstances in which stenting across the inguinal ligament is required, a self-expanding stent is recommended, given its resistance to being irreversibly crushed. Internal iliac artery insufficiency may lead to problems with impotence, and patency should be maintained. However, stenting across the internal iliac artery origin is often required and is usually not associated with adverse outcomes (Fig. 35-6). The internal iliac artery has been shown to remain patent despite stent coverage in 89% to 100% in prior reports.43,44 Internal iliac artery occlusions immediately after stenting are usually the result of overlapping stents in the area of the origin of the internal iliac artery. A recent study showed no new cases of impotence with aortoiliac stenting, unlike in the bypass cohort (where the reported rate of impotence was 28%).45 Nevertheless, if technical success can be achieved with sparing of the origin of the internal iliac artery, the origin should be spared. CIA ostial lesions will be discussed later. As mentioned earlier, numerous stents are available for iliac revascularization, although most qualify as off-label use.46 Given similarities between current stents, few factors besides personal preference dictate stent selection. Self-expanding stents come in longer lengths, allowing use of a single stent for diffuse disease even for very long lesions. Conversely, focal, ostial, and lesions with high elastic recoil, such as eccentric and calcific stenoses, may predicate use of a balloon-mounted stent for precise placement and greater hoop strength. Contralateral delivery of stents is now no longer limited to self-expanding stents. Improved trackability allows contralateral placement of newer-generation balloon-mounted stents, unlike the first-generation Palmaz stent that could be problematic if delivered over the bifurcation because of its rigidity. Choice of an appropriate stent depends on the lesion morphology and location. In tortuous vessels, self-expending stents assure good flexibility and vessel conformability. Nitinol stents are appropriate for treating vascular segments with abrupt transition in size. Covered stents are usually reserved for treatment of isolated iliac aneurysms, iatrogenic perforation, ruptures, and arteriovenous fistulas. More studies are needed on their primary use in aortoiliac occlusive disease.10 Kissing stents (Fig. 35-7) are usually done when atherosclerotic plaque involves one or both CIA ostia and extends proximally to involve the terminal aorta.47–49 It is our practice however to not reconstruct the aortic bifurcation more than a few millimeters proximally. Aortic stenosis often involves the segment caudal to the inferior mesenteric artery (IMA), and it is usually our practice to place a balloon-expandable stent in that segment, then treat the CIAs separately, if possible, even if a small gap (1-2 mm) is left unstented between the aorta and CIAs. When ostial CIA stenosis is unilateral and does not involve the terminal aorta, usually a single balloon-expandable stent can achieve the desired result without the need to stent the nonstenotic CIA. Smith et al. reviewed a series of 175 patients with unilateral ostial CIA lesions who were treated with PTA or stenting unilaterally and without contralateral protection. On follow-up, mild non–hemodynamically significant stenosis in the unprotected contralateral CIA was reported in 2 of the 175 treated patients. The authors concluded that protection of the contralateral CIA during PTA or stenting of a proximal CIA lesion is not mandatory.50 There is controversy about outcomes after kissing stent placement. Siskin et al. found that younger age is not a significant risk factor for iliac artery stent failure.51 In a review of 68 patients who underwent kissing iliac stents, however, Yilmaz et al. found that age younger than 50 years was a predictor of lower patency.49 In a retrospective review of 66 patients who underwent endovascular aortoiliac reconstruction, Sharafuddin et al. found that female gender, presence of occlusions, and residual stenosis were predictors of restenosis.48 A few studies showed worse outcomes when treating extensive aortoiliac disease (TASC C/D lesions) with kissing stents compared to less extensive disease. In a retrospective review of 173 patients treated with kissing stents, Björsens et al. found no difference in outcome in patients with TASC C/D compared to TASC A/B lesions, however.52 A few studies found geometric mismatch to be a significant predictor of decreased patency.47–49,52 Geometric mismatch is defined by nonconformities between the native aortic lumen and the final configuration of the stents following aortoiliac reconstruction. Determinants of mismatch include aortic anatomy and configuration (straight vs. tapered shaped) and the stenting geometry itself, which is a reflection of stent material and sizing and stenting configuration.48 If “double-barrel” stents are extended into the distal aorta, a configuration that is not favored in our practice, the dead space between the stent pair and the aortic wall can be reduced by using oversized nitinol self-expanding stents, which can allow a mirror-D configuration and reduce dead space (Fig. 35-8).48 Greiner et al. found a primary patency rate (PPR) and assisted primary patency rate (APR) at 2 years significantly higher for the iliac stenting group without stent extension into the distal aorta (94.1% and 100%, respectively) than those in the group with a double-barrel stent configuration (33.2% and 45.3%).53 It is generally recommended that the proximal end of a stent should not extend more than 0.5 cm over the aortic bifurcation.49 The results are particularly poor when stents extend into the aorta far enough to overlap at least half the width of a stent.54 In the aortoiliac region, like elsewhere in the body, stent type is usually chosen based on lesion characteristics, location, vessel tortuosity, and stent profile. Other factors, however, such as ease of deployment, familiarity of the physician, cost, and availability, are also considerations.49 Self-expanding stents are typically used in longer tortuous lesions. Balloon-expandable stents are commonly used in shorter, more calcified lesions and unilateral or bilateral CIA ostial lesions.48,52 In very calcified lesions with predicted high risk of rupture, primary use of a covered stent can be considered. It has been hypothesized that reconstruction of the aortic bifurcation with covered stents may provide better laminar flow, decreased thrombogenicity, less chance of prolapse of plaque through the covered stent, and less ingrowth of hyperplastic tissue compared to bare metal stents.54 In a retrospective review of 54 patients with aortoiliac disease who received reconstruction with kissing stents (26 with covered stents and 28 with bare metal stents) Sabri et al. found higher primary patency in the covered stent group (92% at 1 and 2 years) compared to the bare metal stent (78% and 62% at 1 and 2 years, respectively).54 Several studies demonstrated the efficacy of using stent-grafts for aortoiliac disease, with overall primary patency ranging from 70% to 85% at 1 year. Limitations to stent-grafting include larger introducer sheaths, costs, and lack of U.S. Food and Drug Administration (FDA) approval.54 Chronic occlusions can represent a technically challenging subset of aortoiliac disease (Fig. 35-9). Long-term outcome of stents for chronic iliac artery occlusions rivals those of bypass surgery, with less morbidity and mortality.41,55–57 When faced with a chronic arterial occlusion, identification of the true lumen may be challenging if not impossible. Injection of contrast medium directed at the occlusion may reveal a sliver of occluded true lumen. Probing with a spring-coil guidewire initially is often successful. If occlusion traversal is not readily achieved, hydrophilic guidewires are often used. In a long-length occlusion, partial traversal from either direction only may be possible. In such cases, a snare introduced into the occluded segment often can be used to pull the other guidewire across from the opposite direction.58 Once the occlusion is traversed, stents are deployed to restore a patent lumen to a desired diameter. Thrombolysis may theoretically facilitate successful revascularization by facilitating traversal, reducing potential distal embolization, and improving stent apposition to the arterial wall. This may hold true for fresh thrombus superimposed on a stenosis or short occlusion. Unfortunately, chronic occlusions are often resistant to thrombolysis, in which case, thrombolysis only adds to procedural time, prolongs hospitalization, and exposes the patient to potential bleeding complications. In a retrospective review of 25 patients with iliac disease with subacute (1 week to 3 months) and chronic (>3 months) symptoms of claudication who underwent thrombolysis prior to intervention, treatment was completed in 21 patients with complete recanalization of the iliac axis, and the underlying lesion was clearly identified in 20 out of 21 patients (95%), and occlusions were transformed into stenosis.59 The complication rate was 8% and consisted of peripheral embolization. Thrombolysis can turn a long occlusion into a shorter and more treatable stenotic lesion, allowing less extensive stenting.59 Martinez and Diethrich reported 100% technical success with a preliminary thrombolysis before PTA and stenting in 6 and 7 patients, respectively. Some authors recently recommended primary stenting without thrombolysis. Their argument was that thrombolysis may increase cost, time, and complications such as distal embolization. Distal embolization, however, can still occur even in the absence of thrombolytic use. The exact role of thrombolysis in chronic aortoiliac disease is still unclear.60 It may be useful in reducing the length of stented segments in those with long occlusions, such as those involving the entire infrarenal aortic segment. Subintimal angioplasty (SIA) was first described by Bolia in 1989. Usually this technique is used for infrainguinal occluded arteries. Often when crossing iliac artery CTO, an intramural path is used; the “true” lumen has long been obliterated. This is often addressed by stenting after lesion traversal. Although early descriptions of angioplasty for chronically occluded iliac arteries were not positive,61 subintimal angioplasty alone has been described as a definitive treatment for chronic occlusions in the iliac arteries. Meta-analysis of SIA analyzing 37 studies of SIA found a technical success rate of 86%, 1-year PPR of 56%, and limb salvage rate of 89%.62 Only two of these studies included iliac artery interventions, for a total of 79 patients. Technical success rates, limb salvage rates, and primary and secondary patency rates are high, as shown by the recent series of stent-supported SIA in the treatment of CTO of the iliac arteries.62,63 Intraluminal reentry can be challenging. It is critical to reenter the true lumen beyond the occlusion but below the aortic bifurcation. Often this may be a challenging task and may require additional maneuvers. Steerable catheters can redirect the wire into the true lumen. Alternatively, using an occlusion balloon as a target, the stiff end of a guidewire can be used to poke into the true lumen. Use of a needle aimed toward an occlusion balloon also has been successful but is rarely required.29 Although sharp recanalization can be successfully accomplished, steps should be taken to ensure safe passage. Distance between the needle and the occlusion balloon should be minimized. Indentation of the occlusion balloon should be noted before an attempt at needle advancement. The occlusion balloon should be sufficiently inflated to avoid collapse of the intimal flap. This is confirmed by conformity of the occlusion balloon to the luminal shape owing to its compliance. Lastly, contrast medium should be injected along the tract once passage has been created to exclude arterial perforation and stenting open an arterial rent (Fig. 35-10). Initial reports of SIA recommended against anticoagulation until after the occlusion was crossed to reduce the risk of bleeding in case of perforation. The risk of thrombus formation in the subintimal space is higher, with subsequent risk of embolization, so anticoagulation is recommended prior to recanalization.64 During subintimal recanalization, it is very important to monitor the behavior of the hydrophilic wire under fluoroscopy. After initial penetration into the subintimal plane, the wire may initially spiral; this is not the preferred path of the wire. Rather than intentionally dissecting where the hydrophilic guidewire assumes a spiral or serpentine configuration, it is usually best to keep the tip free and rapidly rotate the guidewire, probing for the best path. Once the wire is near the end of the occlusion, the catheter is used to straighten the wire which is then advanced freely into the distal lumen in case of true lumen reentry. Another method of penetrating into an occlusion is to use the back end of a standard Glidewire.64 This technique can be used in locations where the direction of the wire exiting the catheter is in the direction of the axis of the occluded vessel. The wire is advanced only a short distance, the catheter is advanced to that point, and the soft end of the wire is again used to cross a remaining occlusion.64 If reentry into the true lumen is not possible distally, centering the wire in the distal portion of the occlusion using an angioplasty balloon can be performed, allowing for support to penetrate back into the true lumen. Inflating a balloon can also cause disruption of the distal cap of the occlusion and allows reentry. If reentry is still not possible, extending the dissection beyond the end of the occlusion may allow reentry, but this may affect branch points and collaterals in areas not otherwise involved and is usually discouraged, especially in the distal aorta, where creation of a dissection that propagates proximally is possible. To avoid this, reentry catheters can be used.64 The Outback LTD catheter (Cordis, Miami Lakes, FL) is a 6F compatible single-lumen system guided over a 0.014-inch guidewire. Initially the wire emanates from its distal tip. The catheter has also a hollow nitinol curved needle that can be advanced through a side hole at the tip. When the device is in the subintimal space, the needle is retracted inside the catheter, and the wire is withdrawn back inside the catheter proximal to the needle tip. The nosecone at the catheter tip has an L-shaped radiopaque marker that indicates needle direction, with the tip of the horizontal limb of the L indicating the direction of entry into the vessel. The marker becomes a T when the fluoroscope is rotated 90 degrees, and the horizontal position of the T should be overlying the artery. The needle is deployed once the catheter is in line with the vessel (T marker on top of the vessel and L marker toward the artery) through the intima and into the lumen. Usually multiple attempts are required. The 0.014-inch wire is advanced into the true lumen and the Outback catheter removed (Fig. 35-11). In a retrospective review of 51 patients with CTO (inflow, outflow, and runoff disease), Aslam et al. showed a technical success rate of 96.1% when using the Outback catheter for true lumen reentry following subintimal recanalization.65 Small series that focused on aortoiliac occlusions showed a technical success of 91% to 100%.66–68 Very few reports of long-term patency following recanalization with Outback exist. Abisi et al. reported a patency rate of 89% at a mean follow-up of 13 (±11 months) in 48 patients with aortoiliac disease where the Outback was used.69 A disadvantage of this device is that it usually requires multiple needle passes, which is relatively innocuous in the superficial femoral artery (SFA) but carries a potential risk of clinically important hemorrhage in the aortoiliac territory.64,69 The Pioneer catheter (Medtronic, Santa Rosa, Calif.) is a 7F compatible dual-lumen monorail catheter that tracks over a 0.014-inch guidewire. It has a 64-element phased-array 20 MHz intravascular ultrasound (IVUS) transducer that works in combination with the Volcano In-Vision Gold IVUS console (Volcano, San Diego, Calif.). This helps identify the blood flow in the true vessel lumen via a color flow mode (Chroma Flo feature). The second lumen of the catheter contains a curved needle that can be deployed and retracted through the side port just proximal to the IVUS transducer. The side port is fixed at the 12 o’clock position on the IVUS image. Once the catheter is in the subintimal space at the level of the presumed reentry site, the device is rotated to place the true lumen at 12 o’clock, and the needle is then deployed across the intimal membrane separating the false and the true lumen. Another 0.014-inch guidewire is then passed into the true lumen.64,70 The technical success rate using the Pioneer catheter is reported to be between 95% and 100%.71,72 In a retrospective review of 11 patients with CTO of the iliac arteries where a Pioneer device was used, the success rate was 100%. At a mean follow-up of 10.5 months, all patients had palpable femoral pulses, with improvement of their ABIs and their symptoms; amputation-free survival was 100%.72 An advantage of the Pioneer catheter is its ability to show the true lumen, limiting the number of needle passes and potentially the risk of perforation. Presence of calcifications can limit the IVUS imaging. The catheter requires a 7F sheath at minimum and will not track in sharply angulated bifurcation owing to the 0.014-inch monorail delivery system and size and rigidity of the transducer at the end of the catheter. Other drawbacks are catheter cost and requirement for an available IVUS machine.64,73 Classically, the presence of significant (>50% stenosis) concomitant disease of the CFA determined whether to proceed with percutaneous endovascular therapy or an open surgical approach. Nowadays, hybrid repair has become an acceptable alternative to total open surgical repair for patients with iliofemoral occlusive disease; aortoiliac disease is treated endovascularly and femoral disease is treated with an open approach such as endarterectomy and patch angioplasty. In a retrospective review comparing hybrid repair with open repair in patients with severe iliofemoral occlusive disease, Piazza et al. showed that hybrid repair has similar early and long-term efficacy when compared to open repair, with shorter hospitalization, and should be considered in all patients with iliofemoral occlusive disease. Long-term patency was similar between the two groups regardless of lesion severity. Recently, treating CFA disease with an endovascular approach (angioplasty, atherectomy, etc.) is gaining acceptance in certain situations. It is often useful to angioplasty the CFA if needed, which usually responds well in our experience.27,74
Aortoiliac Revascularization
Epidemiology
History
Indications
Contraindications
Equipment
Technique
Preprocedure Patient Evaluation
Noninvasive Imaging
Duplex Arterial Mapping
Computed Tomographic Angiography
Magnetic Resonance Angiography
Diagnostic Arteriography
Intervention
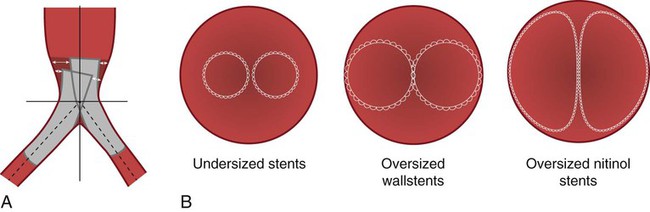
Aortic Bifurcation
Chronic Occlusions
Role of Thrombolysis
Subintimal Recanalization
Reentry Catheters
Outback
Pioneer
Evaluation for Common Femoral Artery Disease
Aortoiliac Revascularization