Fig. 23.1
Digital subtraction angiography (a) provides a more accurate characterization of vessel lumen and calcification (arrow) than routine angiography (b) in this patient with severe, calcified distal right common femoral artery disease
A complete angiographic study includes an image of the entire aortoiliofemoral tree, with additional assessment as necessary. The initial image is best obtained in the anteroposterior (AP) projection, with a field of view large enough to include the aortic bifurcation and the femoral heads (Fig. 23.2). A power injector is often used along with a sidehole catheter (pigtail, straight flush, etc.) placed just below the renal arteries, though hand injection also provides reasonable imaging if DSA is used. A 50/50 mixture of contrast/saline is adequate and is injected at 10 cc/s for 2 s.
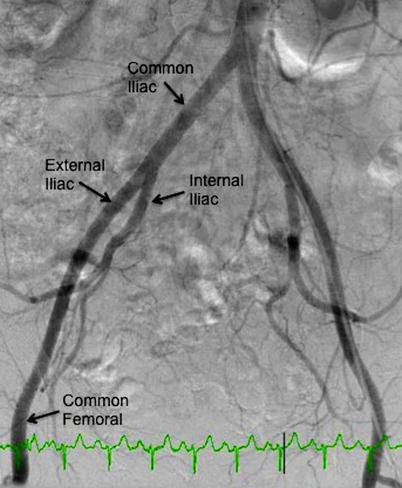

Fig. 23.2
Aortoiliofemoral system (AP projection)
To more closely interrogate the iliac system, especially the bifurcation of the external and internal iliac arteries, contralateral oblique views should be obtained (i.e., LAO projection to visualize the right iliac system) (Fig. 23.3). Injection can be accomplished using a catheter placed at the ostium iliac contralateral to the sheath insertion or with a catheter placed in the midportion of the common iliac artery ipsilateral to sheath insertion. If a more clear delineation of the common femoral system is needed, the ipsilateral oblique view should be obtained (i.e., RAO projection for the right common femoral) (Fig. 23.4).
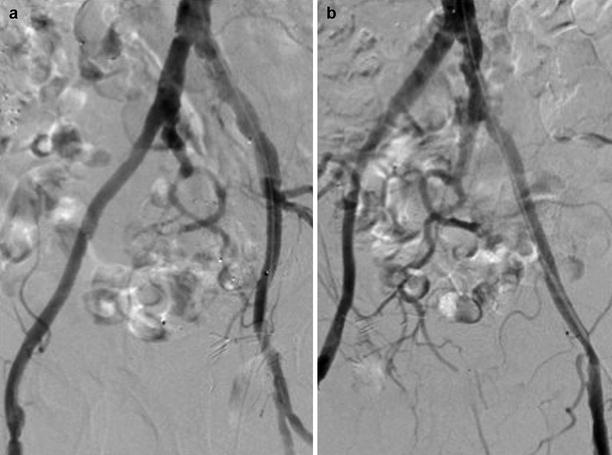
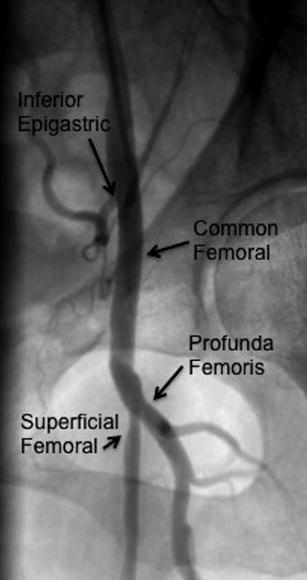

Fig. 23.3
Contraleteral oblique views of the iliac system. (a) LAO projection of the right iliacs. (b) RAO projection of the left iliacs

Fig. 23.4
Left common femoral artery (LAO projection)
Angiographic Assessment
While angiography was once the reference standard for pre-TAVR assessment, it has been since supplanted by 3-D imaging modalities such as MDCT. Nevertheless, since most patients with symptomatic, severe AS present for coronary angiography, simultaneous pelvic angiography can delineate the appropriate subsequent treatment pathway. The angiogram must be assessed for vessel diameter, calcification, and tortuosity. If patients meet clearly meet criteria for TF-TAVR, further evaluation may not be necessary, thereby avoiding costly testing that also requires additional time, radiation exposure, and contrast dye utilization. Similarly, patients with obvious limitations to the TF approach may be directed toward a work-up for alternative access or surgical AVR. Certainly, a number of patients may not fall in to either group, requiring additional evaluation with IVUS and/or MDCT.
Vessel Size
The requirements for vessel size (MLD) are delineated above. Operators in Europe and Canada use the SAPIEN XT or CoreValve, each of which requires in MLD of 6 mm. In the USA, operators still use the first-generation SAPIEN device for commercial implantation, which requires an MLD of 7 mm for the 23 mm valve and 8 mm for the 26 mm valve. In addition to a qualitative assessment of iliofemoral size, precise measurements can be made using quantitative software that is available in most catheterization laboratories (Fig. 23.5). In patients with borderline size, evaluation with IVUS can be considered (discussed below).
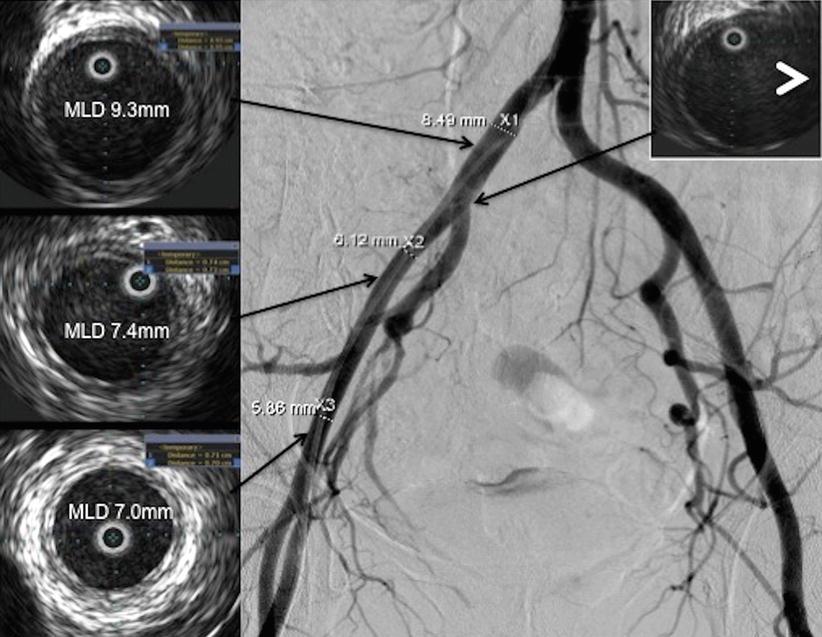

Fig. 23.5
IVUS imaging of the iliofemoral tree. Representative sections confirm adequate lumen diameter for TF-TAVR. Anatomic localization is facilitated by the identification of side branches on the IVUS (white arrowhead)
It should be noted that although the instructions for use (IFU) suggest relatively liberal MLD sizing criteria, more restrictive criteria for sizing, especially early in the learning curve for implantation, is reasonable. Furthermore, patients with a calcified iliofemoral system require an even greater MLD to perform the procedure safely. Hayashida and colleagues demonstrated that a novel indicator, the sheath-to-femoral-artery ratio (SFAR), was a stronger predictor of major vascular complications with TF-TAVR than the size of the arteries or sheath alone, with a threshold value of >1.05 predictive of major vascular complications [2]. In their analysis, when the SFAR was taken together with calcification, the MLD for noncalcified vessels was 6.5 and 6.8 mm for 18- and 19-Fr sheaths, respectively, and 7.2- and 7.5 mm for calcified vessels. This distinction, that a larger lumen is necessary for safe insertion in the presence of calcification, is an important point to remember when assessing MLD for TF-TAVR.
Vessel Tortuosity
Tortuosity of the pelvic vessels is assessed using the following well-accepted scoring criteria: (0) no significant angulation (no tortuosity); (1) 30–60° angulation (mild tortuosity); (2) 60–90° angulation (moderate tortuosity); and (3) >90° angulation (severe tortuosity) [3]. It is generally accepted that angulation <90° is reasonable for TF-TAVR.
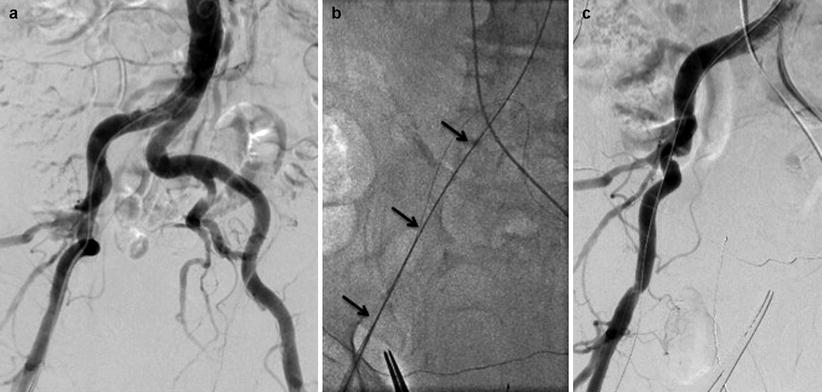
If the pelvic angiogram shows significant tortuosity, it may be reasonable to advance a stiff wire (Amplatz Super Stiff wire or Lunderquist wire, Cook Medical, Bloomington, IN) to the aorta to determine whether the tortuosity can be straightened (Fig. 23.6). If so, TF-TAVR may still be possible. It is important to realize, though, that the straight appearance of the stiff guidewire does not necessarily imply that the vessel itself is straight; rather, the wire takes a “straight” path by apposing to the inside edge of each curve (Fig. 23.6c). Therefore, it is still possible to traumatize the edge of the vessel with the delivery sheath; this is especially true in tortuous vessels with significant calcification. It is also important to remember that when the pelvic angiogram is conducted via femoral access with a pigtail catheter in the descending aorta, tortuosity on the access side may be underestimated due to the straightening effect of the catheter itself.