, Andreia Cristina de Melo1, Angélica Nogueira-Rodrigues1, Gustavo Guitmann1, Gustavo Iglesias1, Julia Alena Leite1, Márcio Lemberg Reisner2, Mariane Sousa Fontes Dias1, Rachele Grazziotin1 and Carlos Gil Ferreira Moreira1
(1)
Brazilian National Cancer Institute, Rio de Janeiro, Brazil
(2)
Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
19.1 Introduction
Cervical cancer represents the third most commonly diagnosed cancer and the fourth cause of cancer death in women worldwide [1]. In 2008, across the world, 530,000 new cases were diagnosed with 275,000 deaths, and this number is expected to increase to 410,000 by 2030 [2, 3]. In the United States, it is the third most common gynecologic cancer diagnosed and cause of death among gynecologic cancers [4]. Human papillomavirus (HPV) is central to the development of cervical neoplasia and can be detected in 99.7 % of cervical cancers [5].
19.2 Epidemiology and Staging of Invasive Cervical Cancer
The incidence and mortality rates of cervical cancer are dependent upon screening programs; the most common strategy employed has been cytological screening using the Papanicolaou (PAP) smear test and Human Papilloma Virus (HPV) vaccination. HPV infections are causally linked to cervical cancer and probably the introduction of HPV vaccines will have an impact on cervical cancer control programs [6]. Due to these interventions, there has been a 75 % decrease in the incidence and mortality of cervical cancer over the past 50 years in developed countries [7]. Currently, developing countries are responsible for 76–86 % of the new cases and 52 % of the mortality rate, ten times higher than in developed countries [8].
Socio-economic position refers to social and economic factors, such as education level, income or wealth, which influence the position an individual or group holds within society. Inequalities in the use of cervical cancer screening services due to socio-economic position have been detected in some settings, with more deprived women less likely to be screened [9]. This difference can be observed in the statistics of developed and developing countries, women in high compared with low poverty countries had a 71 % increase rate of cervical cancer mortality. From 1988 to 1992 in the United States, cervical cancer incidence was higher in women who lived in communities with higher poverty levels (≥20 % or more of the population below the poverty level: 19.2 cases per 100,000 women versus <10 % below poverty level: 8.8 per 100,000) [10].
In developed countries, cervical cancer is the tenth most common type of cancer in women (9:100,000) and it is not even among the top ten causes of cancer mortality (3.2:100,000) [8]. The US estimate for 2013 is 12,340 new cases of invasive cervical cancer and 4,030 cancer-related deaths, which represents about 1.5 % of cancer deaths in women [4]. Rates are usually increased for certain racial and ethical groups in developed countries; e.g. the incidence and mortality is higher in nonwhite (10.7:100,000 and 4.4:100,000) than in white women (7.7:100,000 and 2.2:100,000) [11].
In developing countries, cervical cancer is the second most common type of cancer (17.8:100,000/year) and cause of cancer-related deaths among women (9.8:100,000/year) [8]. Screening improvements since the 1990s resulted in a decrease in the number of diagnosis of invasive cancer lesions, currently approximately 44 % of the diagnosis is of precursor lesions [12]. In Brazil, it was estimated that there were 15,590 new cases of invasive cervical cancer for 2014, a rate of 15,33 cases per 100,000 Brazilian women [13].
Cervical cancer screening can detect early changes that if left untreated can lead to invasive disease. Usually early stages are asymptomatic, once again emphasizing the importance of screening. The aim is to identify abnormal cells sampled from the transformation zone (junction of the ectocervix and endocervix), where cervical dysplasia and cancer generally arise [14].
There are two main types of cervical cancers, squamous cell carcinoma that accounts for 80–90 % of the cases and adenocarcinoma which represents 10–20 % of cervical cancer histologies. There has been an increase in adenocarcinoma relative distribution compared with squamous cell carcinoma in developed countries. Adenocarcinoma has significantly lower survival rates compared with squamous cell carcinoma stage to stage, with higher distant failure rates [15].
The risk factors related with this pathology are mainly: early onset of sexual activity and early age of first birth (≤20 years old), lifetime number of sexual partners, a high risk sexual partner (multiple partners or known HPV infection), history of sexually transmitted disease (STD) e.g. Chlamydia trachomatis and genital herpes, history of vulvar and/or vaginal squamous intraepithelial neoplasia (related to HPV infection) and immunosuppression (impairment to clear HPV infection). Other minor risk factors are oral contraceptive use, cigarette smoking and genetic alterations [16].
The clinical presentation of cervical cancer is usually uncharacteristic, most common symptoms are: Irregular and/or heavy vaginal bleeding, post-coital bleeding and vaginal discharge (watery, mucoid, or purulent and malodorous). These are nonspecific findings and may be mistaken for vaginitis or cervicitis. Advanced disease may present with pelvic or lower back pain, which radiates along the posterior side of the lower extremities. Bowel or urinary symptoms, such as pressure-related complaints, hematuria, hematochezia, or vaginal passage of urine or stool, are uncommon and also suggest advanced disease [17].
In most asymptomatic women, the diagnosis is made as a result of cervical cancer screening or incidentally upon pelvic examination. Clinical examination is the basis for the International Federation of Gynecology and Obstetrics (FIGO) classification, which is the most widely used staging system. FIGO determines that clinical staging for cervical cancer has advantages, such as: more accessible for low resources setting, easier for assessing locally advanced disease and avoids surgery in women who are not candidates for surgical treatment [18].
The clinical assessment of FIGO classification focuses on determining tumoral extension; tumor size, vaginal and/or parametrial involvement, and bladder/rectum tumoral extension (Table 19.1: FIGO staging). Cervical cancer can spread by direct extension or by lymphatic or hematogenous dissemination. Direct extension may involve the uterine corpus, vagina, parametria, peritoneal cavity, bladder, or rectum. Ovarian involvement by direct extension of cervical cancer is rare; ovarian metastases occur in approximately 0.5 % of squamous cell carcinomas and 1.7 % of adenocarcinomas. The most common sites for hematogenous spread are the lungs, liver, and bone; the bowel, adrenal glands, spleen, and brain are less frequent sites.
Table 19.1
Staging cervical cancer (TNM and international federation of gynecology and obstetrics [FIGO])
TNM categories | FIGO stages | Definition | |
|---|---|---|---|
Primary tumor (T) | |||
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
Tisa | Carcinoma in situ (preinvasive carcinoma) | ||
T1 | I | Cervical carcinoma confined to uterus (extension to corpus should be disregarded) | |
T1ab | IA | Invasive carcinoma diagnosed only by microscopy. Stromal invasion with a maximum depth of 5.0 mm measured from the base of the epithelium and a horizontal spread of 7.0 mm or less. Vascular space involvement, venous or lymphatic, does not affect classification | |
T1a1 | IA1 | Measured stromal invasion 3.0 mm or less in depth and 7.0 mm or less in horizontal spread | |
T1a2 | IA2 | Measured stromal invasion more than 3.0 mm and not more than 5.0 mm in depth with a horizontal spread 7.0 mm or less | |
T1b | IB | Clinically visible lesion confined to the cervix or microscopic lesion greater than T1a/IA2 | |
T1b1 | IB1 | Clinically visible lesion 4.0 cm or less in greatest dimension | |
T1b2 | IB2 | Clinically visible lesion more than 4.0 cm in greatest dimension | |
T2 | II | Cervical carcinoma invades beyond uterus but not to pelvic wall or to lower third of vagina | |
T2a | IIA | Tumor without parametrial invasion or involvement of the lower one-third of the vagina | |
T2a1 | IIA1 | Clinically visible lesion 4.0 cm or less in greatest dimension with involvement of less than the upper two-thirds of the vagina | |
T2a2 | IIA2 | Clinically visible lesion more than 4.0 cm in greatest dimension with involvement of less than the upper two-thirds of the vagina | |
T2b | IIB | Tumor with parametrial invasion | |
T3 | III | Tumor extends to pelvic wall and/or involves lower third of vagina, and/or causes hydronephrosis or nonfunctioning kidney | |
T3a | IIIA | Tumor involves lower third of vagina, no extension to pelvic wall | |
T3b | IIIB | Tumor extends to pelvic wall and/or causes hydronephrosis or nonfunctioning kidney | |
T4 | IVA | Tumor invades mucosa of bladder or rectum, and/or extends beyond true pelvis (bullous edema is not sufficient to classify a tumor as T4) | |
Regional lymph nodes (N) | |||
NX | Regional lymph nodes cannot be assessed | ||
N0 | No regional lymph node metastasis | ||
N1 | Regional lymph node metastasis | ||
Distant metastasis (M) | |||
M0 | No distant metastasis | ||
M1 | IVB | Distant metastasis (including peritoneal spread, involvement of supraclavicular, mediastinal, or paraaortic lymph nodes, lung, liver, or bone) | |
Anatomic stage/prognostic groups | |||
Stage 0a | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage IA | T1a | N0 | M0 |
Stage IA1 | T1a1 | N0 | M0 |
Stage IA2 | T1a2 | N0 | M0 |
Stage IB | T1b | N0 | M0 |
Stage IB1 | T1b1 | N0 | M0 |
Stage IB2 | T1b2 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage IIA | T2a | N0 | M0 |
Stage IIA1 | T2a1 | N0 | M0 |
Stage IIA2 | T2a2 | N0 | M0 |
Stage IIB | T2b | N0 | M0 |
Stage III | T3 | N0 | M0 |
Stage IIIA | T3a | N0 | M0 |
Stage IIIB | T3b | Any N | M0 |
T1-3 | N1 | M0 | |
Stage IVA | T4 | Any N | M0 |
Stage IVB | Any T | Any N | M1 |
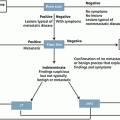
Local expansion to the uterine corpus, vagina, and parametria is the commonest, thus, the cervix and entire vagina should be inspected and palpated to identify overt tumors or subepithelial vaginal extension. Vaginal extension is diagnosed with visual inspection, biopsy is not typically required. Tumor size and parametrial involvement are best assessed by rectovaginal examination. In order to complete staging, basic complementary radiologic imaging is allowed, but not mandatory, e.g. chest X-ray, intravenous pyelogram and radiograph of the skeleton. Assessment of adjacent areas is acceptable using hysteroscopy, cystoscopy, proctoscopy; all suspicious lesions should be confirmed by biopsy. The pathological diagnosis should be made according to the World Health Organization (WHO) Classification based on a surgical biopsy [18, 19].
The limitations of FIGO clinical staging are well appreciated. Parametrial and sidewall invasion, as well as metastases to lymph nodes, can be difficult to assess accurately using the tests listed above. This leads to understaging of some patients. Clinical staging appears to perform best for microscopic or late stage disease, but less well for the stages that depend largely upon assessment of tumor size or local spread [20]. Based upon international data from over 13,000 women with cervical cancer, the correlation between clinical staging and surgicopathologic findings reached 90 % or higher only for stage IA1 (microscopic disease) and stages IIIB and IVA (tumor extends to pelvic sidewall, hydronephrosis, or bladder/rectal invasion) [21]. For other stages, the correlation between clinical and surgical stage ranged from 66 % to 83 %.
Due to limitations of clinical staging system, evaluation with imaging studies, surgical procedures, and laboratory evaluation are routinely used to detect the presence of lymph node metastases and distant metastases. Therefore when available, results of these additional testing modalities should be used for planning treatment, even though, the majority of oncologists will still report their data based upon the FIGO system [19].
It is controversial whether imaging studies are more useful than clinical examination alone to assess tumor size and local spread in women with cervical cancer. If imaging is used, magnetic resonance imaging (MRI) is the modality of choice. MRI is considered the reference complementary imaging modality as it is superior to computed tomography (CT) scan for tumor extension assessment and equal to CT scan for nodal involvement assessment. MRI should be preferred to CT scan and include pelvic and abdominal imaging. Both MRI and CT have low sensitivities for nodal involvement [19].
For women who are surgical candidates based upon clinical staging, some data suggest that tumor size can be determined more effectively with MRI than clinical examination. A prospective study with 208 women, most with stage IB disease, underwent MRI and CT prior to surgery. MRI correlated more closely with surgicopathologic findings than CT or physical examination. All three modalities overestimated tumor size. This is an important observation, as overestimation of tumor size in surgical candidates likely would not change treatment or prognosis, while underestimation of size would potentially triage a patient to surgical excision when chemoradiation would be the best option [22].
The presence or absence of parametrial spread is also of critical importance for determining whether patients are candidates for surgical treatment. There is conflicting data with reference to whether imaging studies are better able to detect parametrial spread than clinical staging. Imaging studies performed better than clinical staging in one study, a prospective multicenter study of 172 women with cervical cancer who were clinically staged as IB or higher underwent CT and MRI prior to surgery [20]. Detection of stage IIB or higher was poor for all approaches, but imaging studies performed better than clinical staging (clinical staging – sensitivity: 29 % and specificity: 99 %; CT – 42 and 82 %; MRI – 53 and 74 %, respectively). If an imaging study is used for parametrial assessment, MRI should be the modality of choice. MRI was found to be superior to CT for evaluation of parametrial involvement in a meta-analysis of 57 studies [23, 24].
There are few data analyzing the use of positron emission tomography PET/CT for the evaluation of tumor size or local spread in cervical cancer. PET has been reported to have sensitivity and specificity of 100 % and 90 % respectively, but it is still under evaluation, and is compared with surgical nodal staging [25].
Surgical pelvic and paraaortic nodal staging are optional. In early stage cervical cancer, sentinel node procedure is currently under study. This technique seems to be feasible method of lymph node assessment with high detection rate, and low false-negative rate, and may even represent a more sensitive procedure than pelvic lymphadenectomy. A literature review including 831 women who underwent lymphatic mapping and sentinel node detection as part of their cervical cancer therapy reported that a sentinel node was identified in 90 % of cases with an overall sensitivity for metastatic disease of 92 % [26].
Sentinel lymph node biopsy appears to perform better than imaging studies. This was illustrated in a meta-analysis of 72 studies including 5,042 women with cervical cancer that evaluated several approaches, and found that the sensitivity and specificity for the detection of lymph node metastases for various approaches were: sentinel node biopsy – sensitivity: 91 % and specificity: 100 %; PET – 75 and 98 %; MRI – 56 and 93 %; CT – 58 and 92 %, respectively [26].
In the presence of nodal metastasis, lymph node dissection may have a therapeutic benefit, and will possibly provide information for treatment planning (to individualize the radiotherapy field). The necessity for and extent of lymphadenectomy (pelvic, paraaortic) depends upon disease stage and imaging findings.
Lymphadenectomy can be performed via laparotomy or laparoscopy through a transperitoneal or extraperitoneal approach. Extraperitoneal and laparoscopic approaches to staging (including extraperitoneal laparoscopic) are associated with reduced morbidity. Potential surgical complications of pelvic and paraaortic lymphadenectomy include vascular damage, ureteral injury, infection, fistula formation, lymphocyst/lymphedema, bowel obstruction, and thrombophlebitis [27].
Historically, obturator lymph nodes were thought to be the most frequent site of nodal metastases. It was also thought that lymphatic spread advanced in an orderly fashion from the lymph nodes on the pelvic sidewall to the common iliac, and then to the paraaortic group. However, subsequent studies, including those utilizing the sentinel lymph node mapping technique, emphasize that any of the pelvic lymph node groups, and even paraaortic lymph nodes, may contain the first draining lymph node and may be the first site of nodal metastasis. This was illustrated in a large retrospective study (n = 619) that evaluated women with cervical cancer patients who had solitary (one or two) positive lymph nodes discovered via radical hysterectomy and complete lymphadenectomy. The distribution of sites of nodal metastasis were: external iliac (43 %), obturator (26 %), parametrial (21 %), common iliac (7 %), presacral (1 %), and paraaortic (1 %) [28, 29].
The risk of pelvic lymph node metastasis increases with increasing depth of invasion, according to the International Federation of Gynecology and Obstetrics (FIGO) staging system:
Stage IA1 – 0.6 %
Stage IA2 – 7 %
The risk of paraaortic nodal involvement increases as the local disease extent increases:
Stage IB – 8 %
Stage IIA – 12 %
Stage IIB – 29 %
Stage IIIA – 17 %
Stage IIIB – 27 %
Stage IVA – 47 %
Although it is a commonly diagnosed disease among women worldwide, there is still a long way to go until optimal screening, staging and management of cervical cancer can be achieved. A broad understanding of the pathogenesis and carcinogenesis can assist technological advances, incorporation of new imaging studies and surgical procedures, therefore improving clinical evaluation and development of a more precise and effective approach to treatment of this disease.
19.3 Pathogenesis
Human papillomavirus (HPV) is central to the development of cervical neoplasia and can be detected in 99.7 % of cervical cancers [30]. It is the single most important etiological agent in cervical cancer, but the infection alone is insufficient for malignant transformation; rather, the virus provides host cells with additional growth stimuli, which extend the proliferative capacity of the infected cell. This implies that HPV oncogenes can override cellular control mechanisms, which in untransformed cells regulate cell cycle progression in response to various antiproliferative signals. Pathogenesis of cervical carcinoma is a multifactorial and multistage process, involving aberrant sequential expression of multiple sets of cellular and viral genes.
There are four major steps in cervical cancer development: infection of metaplastic epithelium at the cervical transformation zone, viral persistence, progression of persistently infected epithelium to cervical precancer, and invasion through the basement membrane of the epithelium [31].
HPV infection is a common sexually transmitted infection which a majority of infected women are able to clear by mounting an effective immune response. Almost 50 % of women will be infected within 4 years after the onset of sexual activity, with prevalence peaking between 25 and 35 years of age. Persistent infections and precancer are established, typically within 5–10 years, from less than 10 % of new infections. Invasive cancer arises over many years, even decades, in a minority of women with precancer, with a peak or plateau in risk at about 35–55 years of age. Each genotype of HPV acts as an independent infection, with different carcinogenic risks linked to evolutionary species [31]. Over 40 types of HPV are known to infect the cervical mucosa, being either low-risk (including 6, 11, 40, 42, 54, and 57) or high-risk types (including 16, 18, 26, 31, 33, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) for cervical cancer [32, 33].
HPV has a double-stranded circularized genome that can be divided into early (E1–E7) and late (L1, L2) open reading frames (ORF). High risk HPV genotypes code for three early proteins (E5, E6, and E7) with cellular growth-stimulating and transforming properties. In productive HPV infection, HPV DNA remains in an episomal state, and the E1/E2 ORFs repress expression of the two most important HPV oncoproteins, E6 and E7 [34]. In contrast, in cervical carcinoma, E1/E2 is frequently disrupted by integration of viral DNA into the host genome, resulting in upregulated overexpression of E6 and E7 [34, 35]. The overexpression of E6 promotes the degradation of the cell cycle regulatory protein p53 through the ubiquitin-mediated pathway, resulting in unchecked cellular progression [32]. By contrast, the E7 oncoprotein binds to and promotes the degradation of the retinoblastoma gene (Rb), resulting in disruption of the Rb cyclin/p16 INK4a cell cycle regulatory pathway [36]. This results in continuous cell proliferation with the increasing risk of accumulation of DNA damage that eventually leads to cancer.
19.4 Pathology
19.4.1 Cervical Intraepithelial Neoplasia
Many systems have been developed for classifying cervical cytologic findings. Although criteria for the diagnosis of CIN and degree of neoplasia vary somewhat between pathologists, the important features of CIN are cellular immaturity, cellular disorganization, nuclear abnormalities, and increased mitotic activity. The term cervical intraepithelial neoplasia, as proposed by Richart [37] refers to a lesion that may progress to invasive carcinoma:
CIN 1 – Mitoses and immature cells present only in the lower third of the epithelium
CIN 2 – Lesions involving only the lower and middle thirds of the epithelium
CIN 3 – Lesions involving the upper third of the epithelium
19.4.2 Comparison of Cytology Classification Systems for Cervical Neoplasms
Following a 1988 National Cancer Institute Consensus Conference, the Bethesda system of classification was developed in an effort to further standardize reporting [38]. This system defines squamous intraepithelial lesions (SILs) as including all squamous alterations in the cervical transformation zone that are induced by HPV; SILs include all lesions that were classified in previous systems as condyloma, dysplasia, or CIN. The Bethesda system divides SILs into two groups: low grade and high grade. Low-grade SILs (LSILs) have nuclear crowding or atypia without frequent mitoses, parabasal cell anisokaryosis, or coarse chromatin; these lesions are usually associated with low-risk HPV types and have a low likelihood of progressing to invasive cancers. High-grade SILs (HSILs) have nuclear atypia in lower and upper epithelial layers, abnormal mitoses, coarse chromatin, and loss of polarity. HSILs are usually associated with high-risk HPV types and have a higher likelihood of progressing to invasive cancer. The Bethesda system was meant to replace the Papanicolaou system and is now widely used in the United States. However, its use is still controversial. Some groups [39, 40] argue that the new nomenclature has failed to improve diagnostic accuracy and believe that with dichotomization of the spectrum of atypical lesions, lesions that were formerly classified as CIN 2 (now HSIL) may be overtreated despite their relatively low risk of progression.
The term atypical squamous cells of undetermined significance (ASCUS) was introduced by Bethesda system. This uncertain diagnosis is now the most common abnormal Pap smear result in United States laboratories [41], with 1.6–9 % of Pap smears reported as having ASCUS. Although most cases of ASCUS reflect a benign process, about 5–10 % are associated with an underlying HSIL, and one-third or more of HSILs are heralded by a finding of ASCUS on a Pap smear.
Histopathologic types of cervical cancer are [42]: squamous cell carcinoma (69 %), adenocarcinoma (including adenosquamous – 25 %) and other histologies (6 %). The incidence of invasive cervical adenocarcinoma and its variants has increased dramatically over the past few decades, particularly in younger women [43, 44]. Several causative factors have been proposed to explain this trend, including increased prevalence of specific HPV-16 and 18 variants that are associated more with adenocarcinoma than with squamous cell carcinoma as well as exposure to estrogens, both endogenous (e.g., obesity) and exogenous (e.g., hormonal contraception, postmenopausal estrogen therapy).
Adenosquamous tumors exhibit both glandular and squamous differentiation. They may be associated with a poorer outcome than squamous cell cancers or adenocarcinomas [15].
19.4.3 Adenocarcinoma In Situ
Adenocarcinoma in situ (AIS) is diagnosed when normal endocervical gland cells are replaced by tall, irregular columnar cells with stratified, hyperchromatic nuclei and increased mitotic activity but the normal branching pattern of the endocervical glands is maintained and there is no obvious stromal invasion. About 20–50 % of women with cervical AIS also have squamous CIN [49]. Because AIS is frequently multifocal, cone biopsy margins are unreliable. AIS is a precursor of invasive adenocarcinoma. It is found adjacent to many invasive adenocarcinomas, often accompanied by squamous dysplasia. Both AIS and invasive adenocarcinoma of the cervix are associated with HPV (usually type 18, but sometimes type 16). AIS is characterized by preservation of the overall endocervical gland architecture. However, endocervical glands and surface epithelium are replaced to varying degrees by cells displaying atypia, including nuclear enlargement and stratification, nuclear hyperchromasia, and mitotic figures. Most adenocarcinomas in situ occur near the transformation zone, and skip lesions are unusual [49].
19.4.4 Squamous Cell Carcinoma
Around 80–90 % of cervical carcinomas are squamous cell carcinomas. Squamous carcinoma of the cervix includes both microinvasive squamous carcinoma and more deeply invasive carcinoma. Small cell squamous carcinomas have small to medium-sized nuclei, open chromatin, small or large nucleoli, and abundant cytoplasm [50]. Sarcomatoid squamous carcinoma is very rare variant, demonstrating areas of spindle-cell carcinomatous tumor confluent with poorly differentiated squamous cell carcinoma; immunohistochemistry demonstrates expression of cytokeratin and vimentin.
19.4.4.1 Preinvasive Disease
Squamous carcinoma in situ is a precursor lesion of invasive squamous carcinoma. Squamous carcinoma in situ is characterized by full-thickness atypia of the cervical epithelium. Endocervical glands may also be involved. The epithelium is replaced by atypical cells that often have enlarged, oval nuclei, increased nuclear-to-cytoplasmic ratios, with mitotic figures.
19.4.4.2 Microinvasive Carcinoma
Microinvasive squamous carcinoma is associated with squamous intraepithelial neoplasia, and may arise from either the surface epithelium or from endocervical glands involved by dysplasia [51]. Microinvasive carcinoma often displays cells that are larger, with more abundant eosinophilic cytoplasm than cells in the adjacent dysplasia. A desmoplastic stromal reaction is usually present. These features are useful in distinguishing microinvasion from rounded, well-circumscribed endocervical glands involved by squamous dysplasia.
19.4.4.3 Invasive Squamous Cell Carcinoma
Invasive cervical carcinoma arises from high-grade dysplasia that may be detected up to 10 years before invasive carcinoma develops. Untreated squamous carcinoma in situ results in invasive carcinoma in about one-third of cases over a period of 10 years. Invasive carcinoma occurs most often after the age of 40 years, although it may be seen in young women. It is associated with human papillomavirus infection in more than 99 % of cases. These tumors may consist of firm, indurated masses, or they may be ulcerated or polypoid.
Mitoses may be numerous, and atypical forms may be present. There is typically a desmoplastic stromal response around the nests of invasive neoplasm. Lymphatic and vascular space invasion may be present, especially in more deeply invasive tumors. Invasive squamous carcinomas are also graded [52], although treatment protocols do not depend on grade, and the histologic grade may not correlate with prognosis. Grade 1 (well-differentiated) tumors are not very common in the cervix. They display keratin pearls and large numbers of keratinized cells. Nuclei display only mild to moderate atypia, and mitoses are typically not numerous. Grade 2 (moderately differentiated) tumors represent the majority of invasive squamous carcinomas of the uterine cervix, and are usually nonkeratinizing squamous carcinomas with nuclear pleomorphism, numerous mitoses, and an infiltrative pattern. Grade 3 (poorly differentiated) tumors either have smaller cells without neuroendocrine differentiation, or are pleomorphic with anaplastic nuclei, and sometimes a tendency to form spindle cells that must be distinguished from sarcoma by positive cytokeratin stains.
19.4.5 Adenocarcinoma
While the incidence of squamous carcinoma of the cervix has decreased in past decades owing to cytologic screening, the number of cases of cervical adenocarcinoma has increased [53, 54]. Adenocarcinoma of various types accounts for 20–25 % of cervical carcinomas [53].
About 80 % of cervical adenocarcinomas are endocervical-type adenocarcinomas, which are composed predominantly of cells with eosinophilic cytoplasm, frequent apoptotic bodies, although many other patterns and cell types have also been observed.
19.4.6 Mucinous Adenocarcinoma
There are several variants of mucinous adenocarcinoma of the cervix, including endocervical, intestinal, signet ring cell, minimal deviation, and villoglandular variants. HPV DNA has been detected in more than 90 % of mucinous adenocarcinomas of the cervix, including endocervical, intestinal, and endometrial subtypes [55]. Endocervical-type adenocarcinomas are frequently referred to as mucinous; however, although some have abundant intracytoplasmic mucin, most have little or none [53].
19.4.7 Endometrioid Adenocarcinoma
Endometrioid carcinomas of the uterine cervix are rare (about 7 % of all cervical adenocarcinomas). These neoplasms display histologic features identical to endometrial carcinoma. Therefore, the possibility of a primary endometrial adenocarcinoma with endocervical extension or drop metastasis must be excluded before establish the diagnosis of primary endocervical endometrioid adenocarcinoma. Immunohistochemistry may help in difficult cases: combination of CEA positivity, ER and vimentin negativity is most often seen in endocervical primary tumors, while the reverse is more often characteristic of endometrial primary tumors. Evidence of association with HPV also supports an endocervical primary neoplasm [56].
19.4.8 Other Adenocarcinomas
19.4.8.1 Clear Cell Adenocarcinoma
Clear cell carcinoma of the cervix has been associated with intrauterine diethylstilbestrol (DES) exposure; however, it also occurs in the absence of DES exposure. Patients usually have a cervical mass. The solid pattern of tumor displays sheets of cells containing abundant glycogen-rich clear cytoplasm, atypical nuclei, and mitoses. The tubulocystic pattern contains tubules and cystic spaces lined by oxyphilic or clear cells. The papillary pattern is the least common variant and often coexists with solid or tubulocystic areas. Clear cell carcinomas of the cervix are not associated with HPV DNA [57].
19.4.8.2 Serous Adenocarcinoma
Papillary serous carcinoma of the uterine cervix has a bimodal age distribution, occurring in patients younger than 40 years and older than 65 years. This age distribution differs from the typical mid-life age of patients with cervical adenocarcinomas in general. Serous carcinomas of the cervix are not associated with HPV DNA [57].
Gross examination may reveal a nodular mass, an indurated cervix, or no visible abnormality. Microscopically, these tumors are identical to serous tumors of the ovary, endometrium, and primary peritoneal serous carcinomas. Considering the rarity with which this type of neoplasm is seen in the cervix, the diagnosis of primary serous carcinoma of the uterine cervix should be made only after excluding metastasis or extension of disease from another site, especially the endometrium [56].
19.4.9 Other Epithelial Tumors
19.4.9.1 Adenosquamous Carcinoma
Adenosquamous carcinoma is a tumor composed of admixed malignant glandular and squamous elements. Adenosquamous carcinomas are more commonly associated with higher tumor grade (p < 0.001) and vascular invasion (p = 0.002) than are adenocarcinomas [58]. Adenosquamous carcinomas appear to be either histologically more aggressive or diagnosed at a later stage than adenocarcinomas of the uterine cervix.
19.4.9.2 Glassy Cell Carcinoma
Glassy cell carcinoma is a rare form of poorly differentiated adenosquamous carcinoma that displays cells with abundant eosinophilic cytoplasm, well-defined cell borders, ground-glass cytoplasm with large round to oval nuclei, prominent nucleoli, and a prominent infiltrate of eosinophils and plasma cells. Occasionally, this morphology may be seen in recurrences of adenocarcinomas or adenosquamous carcinomas that have been treated with radiation therapy [53].
19.4.9.3 Anaplastic Small Cell/Neuroendocrine Carcinoma
Anaplastic small cell carcinomas resemble oat cell carcinomas of the lung and are made up of small tumor cells that have scanty cytoplasm, small round to oval nuclei, and high mitotic activity; they frequently display neuroendocrine features [45]. Anaplastic small cell carcinomas behave more aggressively than poorly differentiated small cell squamous carcinomas; most investigators report survival rates of less than 50 % even for patients with early stage I disease, although recent studies of aggressive multimodality treatments have been somewhat more encouraging. Widespread hematogenous metastases are frequent, but brain metastases are rare unless preceded by pulmonary involvement [59].
19.5 Vaccines
As the knowledge of the role of HPV infection in the natural history of preinvasive and invasive lesions of the lower genital tract was improved, prophylactic vaccination has emerged as an important element in cervical cancer prevention [56]. The aim of prophylactic vaccination is to generate neutralizing antibodies against the HPV L1 and L2 capsid proteins. Prophylactic vaccine development against HPV has focused on the ability of the L1 and L2 virion structural proteins to assemble into virus like particles (VLPs). VLPs mimic the natural structure of the virion and generate a potent immune response [40]. VLPs primarily induce a humoral response with neutralizing antibodies, but they also induce cell-mediated immune responses [56]. Because the VLPs are devoid of DNA, they are not infectious or harmful. HPV VLPs can be generated by expressing the HPV capsid protein L1 in baculovirus or yeast [40]. VLP are combined with different aluminum based adjuvants, which stimulate the immune system and increase the response to vaccination.
It is estimated that if women were vaccinated against all high-risk types of HPV before they become sexually active, there should be a reduction of at least 85 % in the risk of cervical cancer, and a decline of 44–70 % in the frequency of abnormal Papanicolaou (Pap) smears attributable to HPV [5]. Based on the natural history of HPV infection and development of preinvasive and invasive disease, it may take at least 15 years before there is a significant impact on the incidence of CIN 2/3 and perhaps 30 years before there is a change in cervical cancer incidence [56]. Therefore, therapeutic vaccines are still very much needed to reduce the morbidity and mortality associated with cervical cancer.
The therapeutic approach to patients with preinvasive and invasive cervical cancers is to develop vaccine strategies that induce specific CD8+ cytotoxic T lymphocyte (CTL) responses aimed at eliminating virus-infected or transformed cells. The majority of cervical cancers express the HPV-16-derived E6 and E7 oncoproteins, which are thus attractive targets for T-cell–mediated immunotherapy.
Currently, two vaccines are approved in the United States for the prevention of cervical cancer. The quadrivalent vaccine Gardasil (Merck & Co., Inc., Whitehouse Station, NJ, USA) contains VLPs to HPV types 6, 11, 16, and 18 and the bivalent vaccine Cervarix (Glaxo Smith Kline, Rixenstart, Belgium) contains VLPs to HPV types 16 and 18 [56].
Adequate antibody responses have been reported following immunization with quadrivalent and bivalent vaccines [60]. Efficacy studies were restricted to sexually active females, 15 years of age and older. There is no defined minimum threshold titer for protection. Seroconversion from prior exposure has been shown to reduce the risk of incident HPV infection, suggesting that the titers resulting from natural infection, which are lower than those elicited in vaccine studies, provide some level of protection [56, 61].
Quadrivalent HPV vaccine (Gardasil) – Results of two large randomized clinical trials in more than 17,000 adolescents and young females [62, 63] show that among HPV-naive populations, the efficacy for preventing CIN2 or more severe disease due to HPV types included in the vaccine, was 97–100 %. Data collected outside the clinical trial setting are also favorable, demonstrating decreased prevalence of HPV-related cervical disease and genital warts following introduction of quadrivalent vaccine into national immunization programs.
Gardasil is widely available and has been approved in many countries throughout the world for the prevention of cervical, vulvar, and vaginal cancers and their precursor lesions (i.e., cervical, vulvar, and vaginal intraepithelial neoplasia) caused by HPV types 6, 11, 16, and 18 as well as genital warts caused by HPV 6 and 11.
Bivalent HPV vaccine (Cervarix) – A large randomized clinical trial with more than 18,000 young females aged 15–25 years found that [64] among HPV-naive patients, the efficacy of the bivalent vaccine for preventing CIN2 or more severe disease due to HPV types included in the vaccine was 93 %, comparable with the efficacy of the HPV quadrivalent vaccine. All results are consistent with those seen with HPV quadrivalent vaccine. The bivalent HPV vaccine (Cervarix) is widely available and has been approved in many countries throughout the world. This vaccine was also effective against other lesions caused by HPV types 31, 33, and 45, which are closely related to HPV 16 and 18 [56].
19.5.1 Recommendations for HPV Immunization
19.5.1.1 Timing of Immunization
Clinical trial data of vaccine efficacy in males and females suggest that immunization with HPV vaccine is most effective among individuals who have not been infected with HPV, which is also more cost-effective. Thus, the optimal time for HPV immunization is prior to an individual’s sexual debut. Neither vaccine treats [56] or accelerates the clearance of preexisting vaccine-type HPV infections or related disease.
Females who are sexually active should still be vaccinated consistent with age-specific recommendations. A history of an abnormal Papanicolaou test, genital warts, or HPV infection is not a contraindication to HPV immunization [65]. However, immunization is less beneficial for females who have already been infected with one of more of the HPV vaccine types.
All guidelines for HPV vaccination have, as target, the same age group for routine vaccination, but they differ in the catch-up age range. This is primarily due to cost-effectiveness analyses which show the benefit and cost effectiveness is lower when vaccination is given at older ages.
The United States Advisory Committee on Immunization Practices (ACIP), American Academy of Pediatrics (AAP), the American Academy of Family Practice (AAFP), and the American College of Obstetricians and Gynecologists (ACOG) recommend the bivalent or quadrivalent HPV vaccines for females aged 11–12 for the prevention of cervical, vaginal, and vulvar cancer and the related precursor lesions caused by the HPV types targeted by these vaccines [56, 66].
The bivalent or quadrivalent vaccines can be administered to females as young as age 9. Catch-up vaccination is also recommended for females aged 13–26 years who have not been previously vaccinated or who have not completed their vaccine series [67].
The American Cancer Society (ACS) guidelines recommend that HPV vaccination should be routinely offered to females aged 11–12 years; immunization may begin at 9 years of age [68]. However, the ACS recommends catch-up vaccination for females aged 13–18 who have not been previously vaccinated or completed their vaccine series, as there is insufficient evidence to recommend for or against vaccination of females aged 19–26 years.
The World Health Organization (WHO) position paper suggests that girls within the age range of 9–13 years should be the primary target population for HPV immunization [69].
Interest in HPV vaccine efficacy and safety in young males makes possible decrease in transmission of HPV infection to female sex partners.
In a placebo-controlled international trial, the efficacy of quadrivalent HPV vaccine was evaluated among 4,065 males aged 16–26 [70]. The results demonstrated were: efficacy of immunization against the development of external genital lesions and persistence of HPV infection (by HPV 6, 11, 16, or 18 types) was 90 % and 86 %, respectively, among HPV-naive males (no evidence of infection with the relevant HPV vaccine types at enrollment) who received all three doses of vaccine. In contrast, vaccine efficacy was significantly lower among the overall patient population with or without HPV infection at enrollment (66 % for the prevention of external genital warts and 48 % for the prevention of persistent HPV infection).
Cost-effectiveness analyses have suggested that male vaccination is less cost effective than female vaccination [71]. However, the overall cost effectiveness of male vaccination depends on a range of assumptions, such as vaccine efficacy, vaccine coverage of females, the range of health outcomes included, and the effect of HPV-associated diseases on quality of life [72]. For women and men, vaccination becomes increasingly less cost effective with increasing age.
Vaccination of pregnant females – Although neither HPV vaccine contains live virus (is not infectious), use in pregnancy is not recommended because of limited data on safety [66]. HPV vaccines are considered teratogenicity category B [56]. Lactating females can safely receive the immunization [56] series since subunit vaccines do not affect the safety of infant breastfeeding [73].
If a woman receives the HPV vaccine before she knows that she is pregnant she should be reassured that there is no evidence that this vaccine will harm the pregnancy [63]. However, females who have started the series, but become pregnant before completion of all three shots, may resume the series when postpartum.
Vaccination of immunosuppressed or immunocompromised hosts – Transplant recipients and HIV-infected patients, particularly those with low CD4 counts (<200 cells/mm3) are at risk for HPV-related disease.
HPV vaccine is recommended by the ACIP for persons who are immunocompromised as a result of infection, disease, or medications through age 26 years if they have not already received any or all vaccine doses [66].
19.5.2 Immunization Schedule
Quadrivalent vaccine (Gardasil): administered in three doses at time zero, 2 and 6 months of follow-up.
Bivalent vaccine (Cervarix): administered in three doses at time zero, 1 and 6 months of follow-up.
The ACIP recommends that if the vaccination series is interrupted for any length of time, it can be resumed without restarting the series.
HPV vaccines have shown excellent duration of protection for the time periods through which they have been studied. However, the duration of protection after immunization is unknown; to date, women have been protected during a mean follow-up time of 42 months after the first dose of quadrivalent HPV vaccine [74]. The precise level of antibody needed for protection against infection is also unknown.
Challenges for HPV vaccination include older age for vaccination, a three-dose regimen at a high cost relative to other childhood vaccines, and potential socio-cultural concerns about HPV being a sexually transmitted disease [56]. The majority of cervical cancer cases occur in the developing world [56, 75] and patients in these nations are less likely to receive HPV vaccination. Despite its high cost relative to other childhood vaccines, in nations with high incidence, emerging models suggest that vaccination is cost-effective [56, 76].
19.6 Cervical Cancer Treatment
When confronted with initial cervical cancer (IA1 – IIA) the most important clinical decision will be with which radical treatment to initiate by. Deciding whether to pursue surgery Table 19.2 or radiotherapy (the latter typically combined with chemotherapy – cisplatin) is a controversy probably as old as the coexistence of these treatment options.
Table 19.2
Radical Hysterectomy Classification
Rutledge et al. [84] | Querleu and Morrow [85] | Procedure description | Classic indication |
|---|---|---|---|
I | A | Extrafascial simple hysterectomy without important resection of parametria or vagina | Microinvasive cancer |
II | B1 | Radical hysterectomy where the uterine vessels are ligated at the crossing of the ureters as well as the section of the parametria; removal of the upper fourth of the vagina (≥1 cm) | Microscopic tumor or macroscopic ≤2 cm |
N/A | B2 | B1 + paracervical lymphadenectomy | |
N/A | C1 | Radical hysterectomy with section of the parametria at the level of the internal iliac vessels; removal of the upper third of the vagina (≥≥2 cm); nerve sparing | Macroscopic tumor >2 cm |
III | C2 | C1 but without nerve preservation “Wertheim-Meigs” | |
N/A | D1 | Radical hysterectomy with parametria resection extended laterally; resection and reconstruction of one or more internal iliac vessel | Recurrent disease invading the lateral pelvic wall (still undergoing investigation) |
N/A | D2 | D1 + resection and reconstruction of the pelvic wall – muscle and/or bone | |
IV | N/A | Type III or C2 + extensive dissection of the ureter and section of the vesicouterine ligament adjacent to the bladder | Recurrent disease (rarely without prior treatment), with extension to the bladder (historical significance) |
V | N/A | Type III or C2 + partial bladder resection and ureteral single or bilateral reimplantation |
In a trial published in 1997, Landoni randomly allocated 337 patients to be submitted either to radiotherapy (without surgery) or to radical hysterectomy. No statistically significant difference was found in life expectancy between both groups [77]. However, insufficient data regarding these treatment options, especially after the advancements in both fields with the wide adoption of radio-chemotherapy and minimally invasive surgery, compromises direct comparisons. Therefore, the therapeutic strategy for uterine cervical cancer should be decided on an individual basis and determined by factors such as disease extension (estimated by the clinical stage, often established by FIGO – 2009), the patient’s health status (age and comorbidities) and by specific considerations like the desire to preserve fertility.
19.6.1 Stage IA
Stage IA involves microscopic lesions with horizontal or superficial extension and limited vertical invasion with low risk for lymphatic dissemination (less than 1 %) [78]. Lesions classified as stage IA1 by the FIGO system and without angiolymphatic invasion are considered low risk lesions and eligible for a conservative surgical treatment [79]. The indicated surgical treatment in this scenario for women with no desire for future pregnancies is the extrafascial simple hysterectomy, which could be performed through the access that best suits the patient and surgeon’s experience.
A wide cone biopsy (cold knife or LLETZ) could be performed in patients who desire fertility preservation as long as negative margins are obtained.
Treatment for tumors stage IA2 remains controversial. According to literature, up to 13 % of patients in this group may have positive lymph nodes. This relatively high incidence generally contributes to the indication of a more radical approach [80]. On the other hand, a recent literary review [81] suggests an incidence lower than 1 % for lymph node positivity in stage IA2 patients. Historically, there has been a tendency to attribute an unfavorable prognosis to adenocarcinomas, contraindicating any attempt to a non-radical approach. However, recent case studies including one literary review [82], support that micro invasive cervical adenocarcinomas may be treated in the same manner as squamous cell carcinomas when in equivalent stages. The presence of angiolymphatic invasion in pathology reports, regardless of tumor histology or degree of invasion, considerably increases the risk for lymph node metastasis [83] and determines the necessity for a radical approach (refer to stages IB1 and IIA1 below).
19.6.2 Stages IB1 and IIA1
Tumors up to 4 cm in diameter, limited to the cervix or compromising the upper third of the vagina, defines the patient population that most benefits from a radical surgical approach (radical hysterectomy combined with bilateral pelvic lymphadenectomy). Radical hysterectomy is not defined by a single technique but rather by a group of techniques united by a common denominator – removal of the uterus en bloc with the upper third of the vagina. The Rutledge, Piver and Smith classification [84] or even more recent, the Querleu and Morrow classification [85] define different classes of hysterectomies based on the extension of vaginal and parametrial resection with or without preservation of the hypogastric nerve plexus. The purpose of this discussion is not to provide a detailed description of each individual technique due to the variety and complexity of possible procedures and anatomical considerations. The table below summarizes the different classes of hysterectomies and each indication. Refer to the bibliographic references and surgical textbooks for further information. Note that none of the radical hysterectomy techniques described below include oophorectomy as a mandatory procedure. Given the rarity of occult metastatic involvement of the ovaries (<1 %) in initial stages and the benefits of hormone function preservation in young women, ovarian-sparing hysterectomy could be a feasible option. The most frequently adopted procedures are the type II Piver radical hysterectomy (Querleu-Morrow B) and type III (Querleu-Morrow C-1 or C-2), the tumor size generally orienting the most indicated technique (2 cm cut-off). The most significant difference between these procedures, besides operative time, is hypogastric nerve plexus injury clinically manifested as bladder dysfunction, necessity for intermittent or permanent bladder catheterization, recurrent urinary tract infections and diminished quality of life. Less extensive procedures (type II or B) reduce manipulation of the hypogastric nerves, minimizing bladder function disruption. Furthermore, since the original prospective study [86], increasing evidence supports that type II or B radical hysterectomy could be sufficient treatment for lesions limited to the cervix up to 4 cm in diameter (refer to stages IB2 and IIA2 below). Recent studies have questioned the necessity for any degree of parametrial resection, suggesting the permanent substitution of the radical hysterectomy for the simple hysterectomy [87]. However, there is still no consensus that defines the ideal extent of resection, leading many centers to continue indicating type III or C hysterectomies. This practice increases the interest in nerve sparing surgery (type C1) intended to successfully combine surgical radicalness with decreased neurological morbidity [88].
19.6.3 Fertility Preservation
The concept of fertility preservation originally described by Daniel Dargent in 1987 [89], consists of the resection of the cervix, proximal parametria and upper third of the vagina, conserving the uterine body which is anastomosed to the remaining vaginal wall. This procedure, also known as radical vaginal trachelectomy, could be performed via abdominal incision or transvaginal as long as adequately complemented by pelvic lymphadenectomy, preferably through video laparoscopy. Subsequent studies [90] support that this feasible technique respects fundamental oncological principles present in traditional radical hysterectomy while successfully maintaining fertility, occasionally affected by cervical insufficiency or stenosis. The literature is limited regarding the use of this technique for gestation preservation in pregnant women [91]. The success rate for radical vaginal trachelectomy depends on adequate patient selection. Patients who require adjuvant radiotherapy (refer to adjuvant radiotherapy for indications) will experience endometrial and ovarian dysfunction, impairing the possibility for future pregnancies. As in Dargent’s original series, current recommendation for this procedure is limited to patients with tumors up to 2 cm, yet a few studies have questioned this limit and even considered the possibility of neoadjuvant chemotherapy [92].
19.6.4 Minimally Invasive Surgery
The amount of prospective and randomized evidence available comparing video laparoscopic versus conventional hysterectomy is still surprisingly scarce despite the existence of reports on laparoscopy dating back over 20 years [93] and the widespread practice of this procedure amongst the gynecologic oncology community [94]. Yet sufficient retrospective data [95] consistently support the advantages of a minimally invasive access such as blood loss reduction, faster reestablishment of intestinal function, less painkiller use and hospitalization period. Robotic video laparoscopic surgery is a recent technique apparently safe in experienced hands. Success rates are similar to those of conventional surgery [96] yet with improved practical conditions to perform nerve sparing surgery [97]. Other advantages include a smaller learning curve and inferior conversion rate when compared to regular video laparoscopy [98]. Its major limitation is the excessive financial cost, considerably reducing acceptance [99].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree