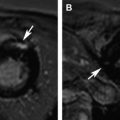
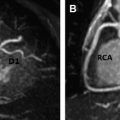
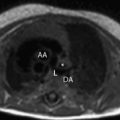
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a rare inherited cardiomyopathy characterized by fibrofatty replacement of the right ventricular myocardium and risk of sudden death from ventricular tachyarrhythmias. Cardiac magnetic resonance (MR) imaging plays an important role in the diagnostic evaluation of patients and family members suspected of having ARVC/D. This article discusses the epidemiology and pathophysiology of ARVC/D, reviews typical MR imaging findings and diagnostic criteria, and summarizes potential pitfalls in the MR imaging evaluation of patients suspected of having ARVC/D. Regional abnormalities of right ventricular (RV) function, such as localized dyskinetic wall motion or microaneurysms, are the hallmark of arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D). Regional wall motion abnormalities and global abnormalities of RV size or function are required to meet major or minor MR imaging Task Force Criteria. Although MR imaging can detect fat and fibrosis in the RV wall in ARVC/D patients, neither of these features are part of the diagnostic criteria, in part because of poor reproducibility and lack of specificity. Left ventricular abnormalities in ARVC/D are common, with both biventricular and left-dominant forms of the disease increasingly recognized. Knowledge of potential pitfalls and mimics in the evaluation of patients suspected of having ARVC/D is important in avoiding misdiagnosis. Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a rare inherited cardiomyopathy characterized by progressive right ventricular (RV) dysfunction caused by fibrofatty replacement of the RV myocardium. Patients have an increased risk of sudden cardiac death from lethal ventricular arrhythmias, which in some cases may be the presenting symptom. Cardiac magnetic resonance (CMR) plays an important role in the diagnostic evaluation of patients and family members suspected of having ARVC/D. This article discusses the epidemiology and pathophysiology of ARVC/D, reviews typical CMR findings and diagnostic criteria, and summarizes potential causes for misdiagnosis in the CMR evaluation of patients suspected of having ARVC/D. ARVC/D is a rare disorder with an estimated prevalence of 1:5000 in the general population. Patients are most often in the second through fifth decade of life with a slight male bias. Patients present with a variety of symptoms ranging from palpitations, syncope, and chest pain to life-threatening arrhythmias. Although rare, ARVC/D is an important cause of sudden cardiac death in athletes. Treatment of ARVC/D relies on prevention of sudden death through prophylactic implantation of an internal cardioverter-defibrillator. ARVC/D is a genetic disorder, although the culprit gene is identified in only approximately 30% to 50% of probands. It is inherited in an autosomal dominant fashion with variable and incomplete penetrance. Five culprit genetic defects have been identified, most of which encode structural proteins crucial in the function of the desmosome, a structure critical to cell-cell junctions. These structural abnormalities result in progressive myocyte death and replacement of the RV myocardium with fat and fibrosis. There is regional variation in the prevalence of ARVC/D-causing mutations; plakophilin-2 (PKP2) is the most common mutation in North American cohorts, whereas in some European cohorts, desmoplakin (DSP) and desmoglein (DSG) are more prevalent. The diagnosis of ARVC/D is challenging for several reasons. Although inherited in an autosomal dominant fashion, ARVC/D has variable penetrance and expressivity. It can present over a wide range of ages with significant differences in phenotypic severity. In one large North American cohort of 108 probands with ARVC/D, age at diagnosis ranged from 12 to 63 years. Screening of family members is particularly challenging, as even if a culprit genetic defect is identified, subjects harboring identical mutations may have dramatic differences in presentations and disease course. Adding to the challenge is the lack of a single specific diagnostic test for ARVC/D. At present, diagnosis is based on a group of clinical, imaging, and histopathologic parameters known as the Task Force Criteria (TFC), which were originally proposed in 1994 and subsequently revised in 2010. The TFC designate a group of major and minor criteria, including personal and family history of sudden cardiac death, electrical abnormalities from electrocardiography (ECG) or Holter monitoring, functional and structural abnormalities of the right ventricle from cardiac imaging studies, genetic profile, and RV histopathology. CMR TFC under the 1994 and 2010 proposals are detailed in Table 1 . Severe dilatation and reduction of RV EF with no (or only mild) LV impairment Localized RV aneurysms (akinetic or dyskinetic areas with diastolic bulging) Severe segmental dilatation of the right ventricle Regional RV akinesia or dyskinesia or dyssynchronous RV contraction And 1 of the following: Ratio of RV end-diastolic volume to BSA ≥110 mL/m 2 (male) or ≥100 mL/m 2 (female) Or RV EF ≤40% Mild global RV dilatation and/or EF reduction with normal left ventricle Mild segmental dilatation of the right ventricle Regional RV hypokinesis Regional RV akinesia or dyskinesia or dyssynchronous RV contraction And 1 of the following: Ratio of RV end-diastolic volume to BSA ≥100 to <110 mL/m 2 (male) or ≥90 to <100 mL/m 2 (female) Or RV EF >40% to <45% Cardiac MR imaging is uniquely suited for the evaluation of ARVC/D, given its strengths in imaging of the right ventricle. Echocardiography, the first-line noninvasive imaging test for cardiac evaluation, is limited for the right ventricle because of its operator dependency and difficulty resolving the RV wall in the near field. CMR is operator independent and provides high spatial and contrast resolution, allowing definitive evaluation of RV function and morphology. Standard CMR ARVC/D protocols typically include cine images for evaluation of cardiac function, dark blood images with and without fat saturation for evaluation of ventricular morphology, and postcontrast late gadolinium enhancement (LGE) images to evaluate for myocardial fibrosis ( Table 2 ). As a component of the TFC, MR imaging plays an important role in the diagnostic evaluation of patients with suspected ARVC/D and is frequently crucial to establishment of the diagnosis. As already discussed, ARVC/D is a difficult diagnosis, and patients may present with nonspecific symptoms such as palpitations or chest pain. Electrical abnormalities are common in clinical practice, and the absence of structural disease at MR imaging is important to differentiate ARVC/D from benign abnormalities such as RV outflow tract tachycardia. MR imaging is useful not only for establishing initial diagnosis of ARVC/D but also for screening of family members at risk. Genetic testing is now routinely performed in the management of patients and family members with ARVC/D. As a result, an increasing number of MR imaging examinations will be performed on subjects identified, based not on symptoms but rather on abnormal genetic testing. A recent 2013 study by te Riele and colleagues found that MR imaging may be helpful in this capacity for identifying gene carriers with a high-risk phenotype. In this study, the combination of both MR imaging and electrical abnormalities in ARVC/D gene carriers identified an advanced disease phenotype that was associated with higher risk of subsequent arrhythmic events, whereas none of the subjects with normal MR imaging experienced arrhythmic events at follow-up. The MR imaging component of the TFC contains 2 elements: (1) qualitative evaluation of regional wall motion and (2) global quantification of RV size and function. Abnormalities in both elements must be present to meet MR imaging criteria. This rule represents an important change from the initial 1994 TFC for which qualitative findings, such as localized aneurysms or segmental RV dilatation, could qualify for major or minor criteria. Quantitative criteria were added with the 2010 revision to limit subjectivity and improve specificity of diagnosis. The effect of these changes was investigated by Vermes and colleagues, who studied 294 patients referred for ARVC/D evaluation, 69 of whom were initially diagnosed with major MR imaging criteria under the 1994 guidelines. After applying 2010 criteria, MR imaging had significantly improved specificity, increasing from 78% by 1994 TFC to 94% with 2010 TFC. However, this came at the expense of reduced sensitivity, with only 4 of 10 patients with ARVC/D meeting major criteria as per 2010 guidelines, compared with 9 of 10 using 1994 criteria. Many fewer subjects met major criteria, and 97% of those previously meeting minor criteria met no criteria at all under 2010 guidelines. Despite these changes to the MR imaging criteria, additional modifications to the clinical portion of the TFC, the most significant of which were changes to ECG criteria, family history criteria, and the inclusion of major criteria points for identification of a pathogenic ARVC/D-causing mutations, has resulted in an overall increased sensitivity for the diagnosis, particularly among family members of probands. Regional abnormalities of RV wall motion are the characteristic imaging signature of RV dysplasia. These regional abnormalities can manifest as areas of akinesia, dyskinesia, or bulging microaneurysms or macroaneurysms ( Figs. 1 and 2 ). Traditionally, ARVC/D in the right ventricle is thought to preferentially involve the RV inflow, outflow, and apical regions, known as the “triangle of dysplasia.” This concept was based on early studies in ARVC/D that were biased toward description of subjects with advanced disease. However, a recent study of gene-positive subjects with ARVC/D, many with early identification through family screening, has brought into question this dogma. In this particular study, 74 patients with ARVC/D underwent detailed cardiac MR imaging evaluation to localize wall motion abnormalities. In patients with limited structural disease, regional wall motion abnormalities of the right ventricle were most often found in the inferior wall, the anterolateral wall, and at the interface between these 2 regions, the so-called RV angle (see Fig. 1 ). Involvement of the RV apex was a finding only found in association with advanced structural disease throughout the entire RV. In the same study, these regions corresponded well with low-voltage scar identified by invasive electroanatomic mapping. Cine steady-state free precession bright blood images obtained in axial, short-axis, and RV outflow tract planes are most helpful for identification of these abnormalities. Inferior wall abnormalities in subjects with early disease are best identified on short-axis images; however, care should be taken to distinguish this from dynamic volume averaging of the right atrium resulting from tricuspid annular displacement during the cardiac cycle. For this reason, confirmation of wall motion abnormalities on contiguous slices is recommended. Axial images are particularly helpful for evaluation of the RV outflow tract (see Fig. 2 ).
Key points
Introduction
Epidemiology and pathophysiology
1994 Task Force Criteria
2010 Task Force Criteria
Major Criteria
Minor Criteria
Role of MR imaging in arrhythmogenic right ventricular cardiomyopathy/dysplasia
Imaging Sequence
T1 Dark Blood
Bright Blood Cine
Delayed Enhancement
Plane
Axial, SAX
SAX, HLA, RVOT, axial
SAX, Axial
Type
FSE with/without fat suppression
SSFP
Segmented GRE
TR/TE (ms)
1–2/30
2.6/1.3
7.2/3.2
Flip angle (°)
180
40–80
25
Slice thickness (mm)
5
8
8–10
Slice gap (mm)
—
2
2
Field of view (cm)
28–32
36–40
36–10
Inversion time (ms)
—
—
150–250
Temporal resolution (ms)
—
30–40
—
MR imaging Task Force Criteria
Regional wall motion abnormalities