Arterial Emergencies
Caitlin Bailey
Daniel Mantuani
Arun Nagdev
INTRODUCTION
The bedside ultrasound examination in the patient with a suspected arterial emergency has continually advanced since its introduction into clinical practice. Initial exams were limited to gray scale measurements of aortic diameters in patients suspected of a leaking abdominal aortic aneurysm, although over time clinicians have begun to recognize the potential value of bedside ultrasound for the rapid detection of aortic dissections and disorders of the peripheral vasculature, and for evaluation of problematic dialysis access. Many emergency physicians have developed technical skills and obtained clinical experience in advanced imaging techniques (color and spectral Doppler), allowing a more thorough evaluation in cases of suspected arterial emergencies. Although these applications are outside the expertise of some, with additional and focused training they are an evolving application that may expedite and prioritize the care of patients with vascular emergencies.
CLINICAL APPLICATIONS
Arterial vascular emergencies present in a number of forms and clinical acuities, but those most commonly evaluated by sonography include: (1) aortic dissection, (2) acute arterial occlusion, (3) pulsatile masses, and (4) nonfunctioning dialysis access. While abdominal aortic aneurysm is also an arterial emergency, it is covered in Chapter 10.
The utility of ultrasound for vascular emergencies lies in its ability to improve diagnostic certainty, expedite care, and prioritize diagnostic tests and specialty consultation for conditions that are notoriously difficult to evaluate. For example, while aortic dissection is a true vascular emergency for which increased mortality has been linked to delay in diagnosis, signs and symptoms suggestive of dissection are unfortunately insensitive (1).  PEDIATRIC CONSIDERATIONS: Aortic dissection is very rare in normal children. However, children with underlying conditions such as Marfan syndrome, Ehlers-Danlos syndrome, collagen vascular disorders and Kawasaki disease are at risk. These children must be evaluated when they present with symptoms typically found only in adults.
PEDIATRIC CONSIDERATIONS: Aortic dissection is very rare in normal children. However, children with underlying conditions such as Marfan syndrome, Ehlers-Danlos syndrome, collagen vascular disorders and Kawasaki disease are at risk. These children must be evaluated when they present with symptoms typically found only in adults. Aortic dissection can present with chest, back, or abdominal pain; syncope, acute neurological deficits, or limb ischemia (2). While most patients have hypertension, some can be deceptively normo- or hypotensive on presentation (2). Given the broad range of clinical presentations, aortic dissection is frequently not the leading diagnosis considered; one small retrospective series reported that emergency physicians initially suspected the diagnosis in only 43% of patients with an ultimate diagnosis of aortic dissection (3). In these challenging circumstances, imaging is required whenever dissection is suspected. Bedside ultrasound can detect findings suggestive of an aortic dissection, which may lead to an earlier diagnosis.
Aortic dissection can present with chest, back, or abdominal pain; syncope, acute neurological deficits, or limb ischemia (2). While most patients have hypertension, some can be deceptively normo- or hypotensive on presentation (2). Given the broad range of clinical presentations, aortic dissection is frequently not the leading diagnosis considered; one small retrospective series reported that emergency physicians initially suspected the diagnosis in only 43% of patients with an ultimate diagnosis of aortic dissection (3). In these challenging circumstances, imaging is required whenever dissection is suspected. Bedside ultrasound can detect findings suggestive of an aortic dissection, which may lead to an earlier diagnosis.
 PEDIATRIC CONSIDERATIONS: Aortic dissection is very rare in normal children. However, children with underlying conditions such as Marfan syndrome, Ehlers-Danlos syndrome, collagen vascular disorders and Kawasaki disease are at risk. These children must be evaluated when they present with symptoms typically found only in adults.
PEDIATRIC CONSIDERATIONS: Aortic dissection is very rare in normal children. However, children with underlying conditions such as Marfan syndrome, Ehlers-Danlos syndrome, collagen vascular disorders and Kawasaki disease are at risk. These children must be evaluated when they present with symptoms typically found only in adults. Aortic dissection can present with chest, back, or abdominal pain; syncope, acute neurological deficits, or limb ischemia (2). While most patients have hypertension, some can be deceptively normo- or hypotensive on presentation (2). Given the broad range of clinical presentations, aortic dissection is frequently not the leading diagnosis considered; one small retrospective series reported that emergency physicians initially suspected the diagnosis in only 43% of patients with an ultimate diagnosis of aortic dissection (3). In these challenging circumstances, imaging is required whenever dissection is suspected. Bedside ultrasound can detect findings suggestive of an aortic dissection, which may lead to an earlier diagnosis.
Aortic dissection can present with chest, back, or abdominal pain; syncope, acute neurological deficits, or limb ischemia (2). While most patients have hypertension, some can be deceptively normo- or hypotensive on presentation (2). Given the broad range of clinical presentations, aortic dissection is frequently not the leading diagnosis considered; one small retrospective series reported that emergency physicians initially suspected the diagnosis in only 43% of patients with an ultimate diagnosis of aortic dissection (3). In these challenging circumstances, imaging is required whenever dissection is suspected. Bedside ultrasound can detect findings suggestive of an aortic dissection, which may lead to an earlier diagnosis.Similarly, acute arterial occlusion can present in a variety of ways, and diagnosis is often delayed. Acute arterial occlusion may present with severe abdominal pain and evidence of distal ischemia in the form of leg pain and paresthesias, weakness, pallor, poikilothermia, and a pulse deficit. Acute worsening of chronic ischemia may present in a more subtle
fashion, with repeat presentations for back, abdominal, or leg pain, with eventual signs of chronic distal ischemia. Aortic occlusion is rare, but when acute may be rapidly fatal without operative intervention. Emergency department (ED) diagnosis of aortic occlusion has been reported in a two-patient series in which ultrasound for abdominal aortic aneurysms revealed echogenic thrombus in the aorta with absence of flow on Doppler (4). One of these patients had chronic occlusion with evidence of collateral formation; the other had an acute occlusion with severe end-organ dysfunction resulting in death.
fashion, with repeat presentations for back, abdominal, or leg pain, with eventual signs of chronic distal ischemia. Aortic occlusion is rare, but when acute may be rapidly fatal without operative intervention. Emergency department (ED) diagnosis of aortic occlusion has been reported in a two-patient series in which ultrasound for abdominal aortic aneurysms revealed echogenic thrombus in the aorta with absence of flow on Doppler (4). One of these patients had chronic occlusion with evidence of collateral formation; the other had an acute occlusion with severe end-organ dysfunction resulting in death.
Ultrasound diagnosis of acute limb ischemia from arterial occlusion distal to the aorta may be of potential use when patients present with a cold, pulseless extremity. Rapid detection of arterial insufficiency by ultrasound, though not a clinical standard, could facilitate further imaging, consultation, and intervention. In cases of embolic disease, bedside echocardiography might also be useful to detect a source for a suspected embolus.
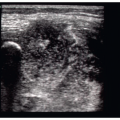
A variety of problems may arise in patients with complications from invasive procedures (such as arterial cannulation or anastomotic grafts) or from trauma. These include hematomas, limb ischemia, pseudoaneurysms, and arteriovenous fistula (AVF) (5). Bedside ultrasound is a useful adjunct to the physical exam when assessing for these complications.
Ultrasound can facilitate the evaluation when there is a clinical presentation suggestive of a vascular access dysfunction, including a reduction of dialysis adequacy (decreased flow rates during dialysis), prolonged needle site bleeding, or pain and swelling over the access site (6). Complications of dialysis access sites cause significant morbidity, decreased quality of life, increased hospitalization, and reduced patient survival in the hemodialysis patient (6). The clinician evaluating renal failure patients should be aware of types of dialysis access and their inherently tenuous nature. Renal failure patients will benefit from a systematic method of both physical and ultrasound evaluation of their dialysis access site.
Lastly, in addition to its role in evaluating diagnostic problems, ultrasound can be used to guide arterial cannulation and has been shown to increase first-pass success, reduce time required for placement, and decrease rates of complications compared with traditional landmark-based techniques (7).
GUIDE TO IMAGE ACQUISITION
Thoracic Aorta
The aortic root, ascending, and descending aorta can be visualized in the parasternal long axis view of the heart. The patient should be supine or in a left lateral decubitus position for this study. A small footprint, low-frequency (2 to 5 MHz) probe is placed in a left parasternal location at approximately the fourth to fifth intercostal space, with the indicator pointed toward the patient’s right shoulder. The aortic root and proximal ascending aorta is seen between the right ventricle and the left atrium. Sliding the probe up higher on the chest wall and directing the ultrasound down can gain a longer view of the ascending thoracic aorta. A cross-sectional view of the descending thoracic aorta can also be seen in the parasternal long axis view posterior to the left ventricle; a rotation of the probe into a parasternal short axis orientation can bring out the long axis of the descending aorta. A similar view can be obtained using a subxyphoid approach. The aortic arch can be viewed from a suprasternal view in which the probe is placed in the suprasternal notch and pointed caudal (8). Descending aortic dissections that extend below the diaphragm and aortic occlusions may be imaged with a standard approach to the abdominal aorta, as described in detail in Chapter 10.
Peripheral Arteries
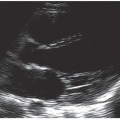
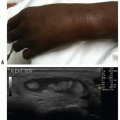
To assess for peripheral arterial occlusion or pulsatile masses, a high-frequency linear transducer is recommended. In some obese patients, a lower frequency transducer may be necessary depending upon the depth of the structure imaged. Image the area of concern in gray scale, looking for abnormalities near vascular structures. Visualization of areas of swelling with color Doppler is then recommended, making sure to begin with a low color velocity scale, and then increasing the scale of the flow in a stepwise manner so that color imaging demonstrates flow only in the area of interest (Fig. 18.1).
Renal Dialysis Access
For the emergency physician, a simplified algorithm to detect gross access dysfunction can be accomplished with moderate practice and a basic understanding of vascular ultrasound. Using the upper extremity evaluation of
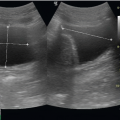
radiocephalic fistula (connection between the radial artery and the cephalic vein) as an example, a stepwise evaluation will allow the clinician to detect access dysfunction. Place the patient in a supine position with the arm relaxed and slightly abducted. The ultrasound screen should be in clear view of the examiner during the exam. Focus on the arteriovenous anastomosis and proximal venous outflow, areas that have been noted to have the highest rate of stenosis. Areas of persistent postdialysis bleeding should also be examined (9). Place a linear transducer in a transverse plane across the arm. Large amounts of ultrasound gel and gentle pressure are important to prevent arterial and especially venous compression. In gray scale/B-mode slowly move the probe distally from 2 cm proximal to the anastomosis (on the arterial aspect). The anastomosis can be difficult to image secondary to the nonlinear directional change. Rotation of the probe in both a clockwise and counterclockwise manner will help in evaluation of the entire anastomosis. This location is often the most difficult aspect of ultrasound imaging, but must be interrogated in detail due to the high prevalence of thrombosis. The proximal venous outflow (approximately the first 2 cm) should also be evaluated in a similar manner (B-mode and then color), looking for areas of focal narrowing and thrombosis. Areas of swelling or postdialysis bleeding should be evaluated in a similar manner (B-mode and Doppler), looking for both vascular luminal narrowing and/ or aneurysmal dilatation. The goal of the focused bedside exam is to identify emergent thrombosis of the access site, not to determine subtle reductions in flow velocities that require evaluation with spectral Doppler (Fig. 18.2) (9).
radiocephalic fistula (connection between the radial artery and the cephalic vein) as an example, a stepwise evaluation will allow the clinician to detect access dysfunction. Place the patient in a supine position with the arm relaxed and slightly abducted. The ultrasound screen should be in clear view of the examiner during the exam. Focus on the arteriovenous anastomosis and proximal venous outflow, areas that have been noted to have the highest rate of stenosis. Areas of persistent postdialysis bleeding should also be examined (9). Place a linear transducer in a transverse plane across the arm. Large amounts of ultrasound gel and gentle pressure are important to prevent arterial and especially venous compression. In gray scale/B-mode slowly move the probe distally from 2 cm proximal to the anastomosis (on the arterial aspect). The anastomosis can be difficult to image secondary to the nonlinear directional change. Rotation of the probe in both a clockwise and counterclockwise manner will help in evaluation of the entire anastomosis. This location is often the most difficult aspect of ultrasound imaging, but must be interrogated in detail due to the high prevalence of thrombosis. The proximal venous outflow (approximately the first 2 cm) should also be evaluated in a similar manner (B-mode and then color), looking for areas of focal narrowing and thrombosis. Areas of swelling or postdialysis bleeding should be evaluated in a similar manner (B-mode and Doppler), looking for both vascular luminal narrowing and/ or aneurysmal dilatation. The goal of the focused bedside exam is to identify emergent thrombosis of the access site, not to determine subtle reductions in flow velocities that require evaluation with spectral Doppler (Fig. 18.2) (9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree