Since acute stroke and transient ischemic attack (TIA) are disruptions of brain hemodynamics, perfusion neuroimaging might be of clinical utility. Recently, arterial spin labeling (ASL), a noncontrast perfusion method, has become clinically feasible. It has advantages compared to contrast bolus perfusion-weighted imaging (PWI) including lack of exposure to gadolinium, improved quantitation, and decreased sensitivity to susceptibility and motion. Drawbacks include reduced signal-to-noise and high sensitivity to arterial transit delays. However, this sensitivity can enable visualization of collateral flow. This article discusses ASL findings in patients with acute stroke and TIA, focusing on typical appearances, common artifacts, and comparisons with PWI.
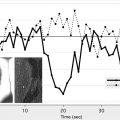
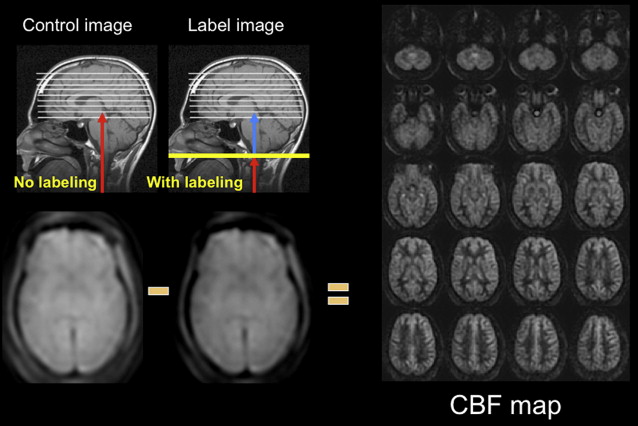
Arterial spin labeling (ASL) is a noncontrast perfusion imaging method that relies on the magnetic labeling of arterial water. This labeling can be performed in a variety of ways; the two major classes of ASL are distinguished from each other based on whether or not the label is applied during a single short-duration pulse (pulsed ASL [PASL]) or during an extended period (continuous ASL [CASL]). Although CASL techniques typically have higher signal-to-noise ratio (SNR), they have been limited in clinical practice by high radiofrequency energy deposition and more demanding requirements from the scanner hardware. Primarily for this reason, PASL techniques were the first to enter routine clinical practice. More recently, however, a hybrid approach, known as pulsed continuous or pseudocontinuous ASL (pcASL), has become popular, in which many short-duration RF pulses can be used to effectively label arterial water over a longer duration. This method combines the high labeling efficiency of CASL with the reduced hardware demands of PASL. Regardless of the labeling approach, images are then acquired with and without labeling after allowing time for the labeled blood to pass from the site of labeling into the tissue of interest. The difference in signal intensity between the two sets of images is approximately proportional to the local cerebral blood flow (CBF) ( Fig. 1 ).
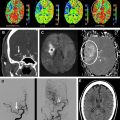
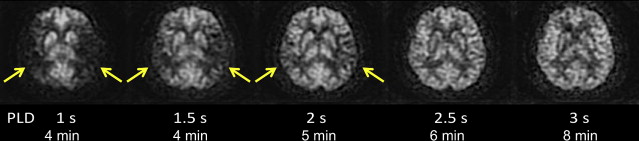
ASL images are sensitive to the exact parameters used to acquire the image, several of which are within the user’s control. Choosing the appropriate labeling time for CASL is often a tradeoff between time and SNR; although longer label times lead to a larger buildup of labeled spins in the voxel, the increased time may be better spent on acquiring additional pairs of label and control images for signal averaging. Postlabel delay (PLD) time, a parameter shared by PASL and CASL, is critical. It defines the time duration for spins to travel from the labeling plane or volume to the imaged slices and is typically between 1500 and 2000 ms. Because ischemic stroke is defined by reduced flow via proximal routes, any remaining flow to the tissue is often supplied via collateral routes, which leads to increased delays between the labeling of the spins and the arrival of those spins in the imaged voxel. Alsop and colleagues demonstrated that longer PLDs lead to improved CBF quantification, although at the price of a loss in SNR; the longer the delay between the labeling and the imaging, the more SNR penalty is paid due to T1-related decay of the labeled spins. Another approach is to obtain images at multiple PLDs, effectively mapping the inflow of label into the tissue ; such approaches offer the potential to quantify arrival times and then fit CBF to kinetic models, although the approach is limited by reduced SNR in each of the individual images, challenges with nonlinear fitting of the inflow curves, and persistent inaccuracies in regions with severely delayed flow. An example of improved CBF quantitation with longer PLD times is shown as Fig. 2 in a patient with bilateral moyamoya disease.
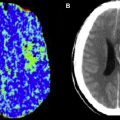
Often, on images in which the arterial arrival times are on the same scale or longer than the PLD, labeled spins are visualized in the arteries feeding the ischemic tissue, a finding termed, arterial transit artifact (ATA). This is particularly evident if no vascular suppression is used; vascular suppression is a method whereby small diffusion gradients are applied to the ASL images so that spins moving above a certain velocity (presumably vascular) are not imaged. ATA, although problematic in terms of quantification, can actually be a useful marker for pathology. ATA in the borderzone regions, often seen bilaterally, is more sensitive than bolus perfusion-weighted imaging (PWI) for identifying subtle perfusion alterations. Also, the presence of ATA in patients with carotid disease is predictive of poor cerebrovascular reactivity after an acetazolamide challenge. One prior stroke study suggested that patients with ATA had improved outcomes and suggested that ATA may represent collateral flow.
ASL differs from the more widely used bolus-based dynamic susceptibility contrast PWI method in several important ways ( Table 1 ). Because of the lack of the use of contrast agents, ASL can be repeated frequently within the same imaging session and, therefore, can be used for challenge-type paradigms. It can give some measure of perfusion in patients in whom gadolinium-based contrast agents are contraindicated due to poor renal function or allergy. As discussed previously, ASL is more susceptible to errors in patients with long arterial arrival times and may overestimate regions of decreased perfusion. Bolus PWI has higher contrast-to-noise per unit time and is an excellent method for measuring cerebral blood volume (CBV), assuming that the blood-brain barrier is intact, but measuring CBF with PWI remains challenging and highly model-dependent. Recently, an approach combining ASL and PWI to more accurately measure CBF has been described that takes advantage of the superior relative perfusion properties of PWI and used ASL to provide a proper scaling factor.
| ASL | Bolus PWI |
|---|---|
| Powerful technique to measure CBF Cannot measure CBV Noncontrast Spin-echo Repeatable Microvascular measurement of perfusion Insensitive to blood-brain barrier damage Errors in tissue with long arrival time 3–5 min scan time | Powerful technique to measure CBV and Tmax CBF challenging Requires gadolinium-containing agents High contrast-to-noise Gradient-echo (primarily) Clinical experience Errors due to blood-brain barrier damage 2 min scan time |
Although the basic method for measuring CBF with ASL was described more than 20 years ago, ASL has only recently entered the clinical arena. The reasons for this are multifactorial. The more widespread use of 3T neuroimaging has led to markedly improved ASL, due to the inherent higher SNR as well as the longer blood T1 at high field. Another important advance was the recognition of the importance of static background tissue signal suppression. To image the spatial distribution of the labeled blood, brain images acquired with and without the label (the control image) must be subtracted from one another. Typically the difference between the label and control images is approximately 1% of the signal intensity of either image. This means that even small amounts of motion between the label and control scans leads to large errors on subtraction images. By suppressing the static tissue signal using multiple inversion pulses, errors associated with patient motion can be significantly reduced; additionally, any biologic or gaussian noise in the individual label and control images is also reduced using this technique, leading to increased SNR of the final CBF images.
Another advance that has facilitated clinical ASL imaging is the ability to use more optimized readout strategies. Traditionally, single-shot echo-planar imaging (ssEPI) has been used to create the label and control images. This was largely because of the high SNR efficiency of this sequence, because the center of k-space is acquired during each acquisition. Many pairs of label and control ssEPI images are collected, and the subsequent difference images are combined using data averaging to improve SNR. This approach, in theory, also allows the elimination of image pairs with patient motion. Once SNR improved and the sequences became less sensitive to motion with the advent of robust background suppression (as described previously), however, it was possible to use other readout approaches, such as steady-state free precession, gradient- and spin-echo (GRASE), and fast spin-echo (FSE). Compared with ssEPI, these methods are more robust in regions with high magnetic susceptibility differences, allowing imaging of structures not typically amenable to ssEPI, such as the posterior fossa, midbrain, and inferior temporal and frontal lobes. FSE imaging also improves performance in patients with aneurysm clips, coils, and blood products. Finally, the use of 3D acquisition methods has entered clinical practice ; the advantages include the acquisition of isotropic voxels, allowing reformatting in different planes, as well as the fact that all slices can be obtained at the same PLD.
Indications for ASL perfusion imaging
Given that bolus PWI has gained some level of acceptance in the clinical arena during the past decade, the question arises as to why one would want to perform a perfusion study using an alternative method, particularly one with less SNR. The recent recognition of the relationship between gadolinium-containing contrast agents and nephrogenic systemic fibrosis has led to both absolute and relative contraindications to such agents at many institutions. In such patients, ASL is the only option for obtaining information about tissue perfusion. Also, intravenous access is challenging in certain patient groups, in particular children and intravenous drug users. Finally, ASL sequences can be easily repeated during a single clinical examination, which is useful for performing cerebrovascular reserve studies using either acetazolamide or breath-hold paradigms.
Specifically, in patients with cerebrovascular disease, ASL has several potential advantages. Although the acquisition time of the study is often a few minutes longer than that of bolus PWI, this is offset by the time-saver of not needing to place an intravenous line or hook up a power injector. Additionally, reconstruction times for ASL tend to be shorter than those for bolus PWI, especially because the background-suppressed ASL variants do not typically require motion correction, as do many bolus PWI studies. ASL excels at identifying bilateral disease, which can occur in this patient group, because it can be used in a quantitative rather than qualitative manner. Finally, the ability of ASL to detect perfusion alterations that are more subtle than those seen with bolus PWI make it an ideal methodology to study minor stroke and transient ischemic attack (TIA), where the ability to ascribe the event to a vascular etiology is of paramount importance, because this may affect treatment triage and outcome.
Previous literature on ASL findings in TIA and stroke
Several early studies documented that ASL imaging could be used to image cerebrovascular disease, such as carotid stenosis and occlusion, as well as acute ischemic stroke. Chalela and colleagues reported on 15 patients imaged within the first 24 hours after acute ischemic stroke. Using CASL, they found perfusion changes in the ipsilateral hemisphere consistent with the diffusion and clinical findings. They found that the quantitative CBF levels in the affected hemisphere correlated with National Institute of Health Stroke Scale (NIHSS) and Rankin Scale at 30-day follow-up. Also, they observed ATA in approximately half of their patients and suggested that this was a good prognostic sign, perhaps reflecting the presence of collateral flow. Detre and colleagues examined the role of ASL imaging with acetazolamide challenge in patients with symptomatic intracranial internal carotid artery (ICA) or MCA stenosis. They showed that the presence of ATA correlated with poor cerebrovascular reactivity after acetazolamide challenge and suggested that ATA could possibly be used to avoid the risks associated with acetazolamide. ASL has also been applied to large vessel disease, as, for example, in a study by Hendrikse and colleagues in patients with unilateral ICA occlusion. They used a PASL method with multiple PLD times, which were then fit to a model to determine CBF in the symptomatic hemispheres of 9 patients and 11 controls. The benefit of their approach was that the results were presumably independent of arrival time, because this is included in the model, although the maximum PLD used (1600 ms) is probably not long enough to ensure that late-arriving flow is appropriately counted. They found a 20% CBF decrease in the hemisphere ipsilateral to the carotid occlusion compared with the contralateral hemisphere or normal subjects.
More recently, Deibler and colleagues, in a series of articles, demonstrated a clinically feasible ASL method and further examined various hypoperfusion patterns. They described the application of a PASL method, called quantitative imaging of perfusion using a single subtraction, second version, which is relatively insensitive to errors associated with slow flow in 3000 clinical patients over a 12-month period. They show examples of the expected CBF changes in the core and penumbra of acute ischemic stroke. They also point out that ASL is not well suited to examine the white-matter perfusion in patients with suspected chronic microvascular ischemia, given the low absolute CBF and the prolonged transit times. Chen and colleagues showed that ASL was useful clinically in 10 pediatric patients with acute ischemic stroke. They also noted that ATA was often present surrounding the ischemic core and tended to be associated with lack of progression to infarct and better clinical outcome.
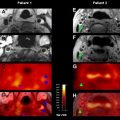
Finally, relatively little has been reported on perfusion in general, and ASL in particular, in TIA patients. A recent study of lacunar syndromes in minor stroke and TIA patients suggested that the presence of a bolus PWI lesion could predict early deterioration (defined as ≥3 points on the NIHSS in the first 72 hours). In the only ASL study specifically focusing on this population, Macintosh and colleagues describes ASL findings in 4 patients with TIA and 11 with minor stroke (NIHSS ≤6). They used a 3D–GRASE-PASL method with multiple PLDs (500–2500 ms) to characterize the CBF and the arterial arrival time (AAT). This attempt to use ASL to measure arrival times quantitatively in stroke patients is significant, given that bolus arrival time markers, such as the normalized time-to-maximum contrast arrival (Tmax), seem to be useful in bolus PWI stroke studies. They proposed an asymmetry index to detect the differences in AAT between hemispheres, and found that AAT was significantly increased in the affected hemisphere. Such an approach, however, is challenging in these patients, because often they have bilateral carotid disease and are abnormal on both sides to some degree. To evaluate this, Zaharchuk and colleagues have begun to systematically examine ASL images in TIA patients with the goal of establishing a vascular etiology for the symptomatology. They have found that approximately 50% of such patients have abnormalities, which exceeds the published yield of bolus PWI in this population (16%). In particular, in the clinically interesting population of 28 TIA patients without diffusion abnormalities, they found that 46% of patients had ASL abnormalities, compared with 18% on PWI, and that all patients with PWI lesions also had ASL lesions. A common abnormality was a bilateral borderzone sign. These findings suggest that ASL alone may be a feasible perfusion method in TIA patients.
Previous literature on ASL findings in TIA and stroke
Several early studies documented that ASL imaging could be used to image cerebrovascular disease, such as carotid stenosis and occlusion, as well as acute ischemic stroke. Chalela and colleagues reported on 15 patients imaged within the first 24 hours after acute ischemic stroke. Using CASL, they found perfusion changes in the ipsilateral hemisphere consistent with the diffusion and clinical findings. They found that the quantitative CBF levels in the affected hemisphere correlated with National Institute of Health Stroke Scale (NIHSS) and Rankin Scale at 30-day follow-up. Also, they observed ATA in approximately half of their patients and suggested that this was a good prognostic sign, perhaps reflecting the presence of collateral flow. Detre and colleagues examined the role of ASL imaging with acetazolamide challenge in patients with symptomatic intracranial internal carotid artery (ICA) or MCA stenosis. They showed that the presence of ATA correlated with poor cerebrovascular reactivity after acetazolamide challenge and suggested that ATA could possibly be used to avoid the risks associated with acetazolamide. ASL has also been applied to large vessel disease, as, for example, in a study by Hendrikse and colleagues in patients with unilateral ICA occlusion. They used a PASL method with multiple PLD times, which were then fit to a model to determine CBF in the symptomatic hemispheres of 9 patients and 11 controls. The benefit of their approach was that the results were presumably independent of arrival time, because this is included in the model, although the maximum PLD used (1600 ms) is probably not long enough to ensure that late-arriving flow is appropriately counted. They found a 20% CBF decrease in the hemisphere ipsilateral to the carotid occlusion compared with the contralateral hemisphere or normal subjects.
More recently, Deibler and colleagues, in a series of articles, demonstrated a clinically feasible ASL method and further examined various hypoperfusion patterns. They described the application of a PASL method, called quantitative imaging of perfusion using a single subtraction, second version, which is relatively insensitive to errors associated with slow flow in 3000 clinical patients over a 12-month period. They show examples of the expected CBF changes in the core and penumbra of acute ischemic stroke. They also point out that ASL is not well suited to examine the white-matter perfusion in patients with suspected chronic microvascular ischemia, given the low absolute CBF and the prolonged transit times. Chen and colleagues showed that ASL was useful clinically in 10 pediatric patients with acute ischemic stroke. They also noted that ATA was often present surrounding the ischemic core and tended to be associated with lack of progression to infarct and better clinical outcome.
Finally, relatively little has been reported on perfusion in general, and ASL in particular, in TIA patients. A recent study of lacunar syndromes in minor stroke and TIA patients suggested that the presence of a bolus PWI lesion could predict early deterioration (defined as ≥3 points on the NIHSS in the first 72 hours). In the only ASL study specifically focusing on this population, Macintosh and colleagues describes ASL findings in 4 patients with TIA and 11 with minor stroke (NIHSS ≤6). They used a 3D–GRASE-PASL method with multiple PLDs (500–2500 ms) to characterize the CBF and the arterial arrival time (AAT). This attempt to use ASL to measure arrival times quantitatively in stroke patients is significant, given that bolus arrival time markers, such as the normalized time-to-maximum contrast arrival (Tmax), seem to be useful in bolus PWI stroke studies. They proposed an asymmetry index to detect the differences in AAT between hemispheres, and found that AAT was significantly increased in the affected hemisphere. Such an approach, however, is challenging in these patients, because often they have bilateral carotid disease and are abnormal on both sides to some degree. To evaluate this, Zaharchuk and colleagues have begun to systematically examine ASL images in TIA patients with the goal of establishing a vascular etiology for the symptomatology. They have found that approximately 50% of such patients have abnormalities, which exceeds the published yield of bolus PWI in this population (16%). In particular, in the clinically interesting population of 28 TIA patients without diffusion abnormalities, they found that 46% of patients had ASL abnormalities, compared with 18% on PWI, and that all patients with PWI lesions also had ASL lesions. A common abnormality was a bilateral borderzone sign. These findings suggest that ASL alone may be a feasible perfusion method in TIA patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree