Currently carotid imaging has 2 main focuses: assessment of luminal stenosis and classification of atherosclerotic plaque characteristics. Measurement of the degree of stenosis is the main assessment used for current treatment decision making, but an evolving idea that is now driving imaging is the concept of vulnerable plaque, which is where plaque components are identified and used to define which plaques are at high risk of causing symptoms compared with those at low risk. This review article covers the methods used for noninvasive assessment of carotid luminal stenosis and the options available for plaque imaging.
Stroke is the third most common cause of death in the United States, with approximately 795,000 new or recurrent events occurring every year, of which 87% are ischemic. Cerebrovascular events impose a significant health, social, and economic burden, with stroke being a major cause of long-term disability that will account for an estimated $73.7 billion of direct and indirect costs in 2010. Carotid atherosclerosis is a significant risk factor for stroke and transient ischemic attack (TIA), with the annual stroke risk from asymptomatic disease being approximately 1.3% and from symptomatic disease (major stroke) being approximately 9.0%. Therefore, investigation of carotid atherosclerosis, as a treatable and preventable cause of stroke, is important in the reduction of this burden.
Current practice in carotid atherosclerosis is to treat either medically or surgically based on the degree of stenosis present and the symptoms. In symptomatic individuals, 3 large randomized controlled trials (North American Symptomatic Carotid Endarterectomy Trial [NASCET], European Carotid Surgery Trial [ESCT], and the Veteran Affairs Cooperative Program Trial) have shown the benefit of carotid endarterectomy compared with medical management in patients with high-grade stenosis (70%–99%). In patients with mild degrees of stenosis (<50% NASCET) the risk/benefit ratio was in favor of medical management. It has also been shown that there is no benefit of operating on individuals who have near occlusive disease. Some benefit for operative intervention in symptomatic individuals with moderate stenosis (50%–69%) was seen. The risk of recurrent events following TIA has been shown to be as high as 9% within the first 7 days, which has led to a more aggressive surgical strategy, designed to treat individuals with operative disease within 2 weeks of symptom onset.
For those patients with asymptomatic carotid disease the decision on when to operate is less clear. The Asymptomatic Carotid Atherosclerosis Study (ACAS) and the Asymptomatic Carotid Surgery Trial (ACST) randomized patients between medical therapy and carotid endarterectomy (>60% stenosis in ACAS, >70% in ACST) with a similar lower rate of stroke/death in the surgical cohort. A more recent review of more than 5000 cases of carotid endarterectomy for asymptomatic disease showed a similar stroke rate between medical therapy and carotid endarterectomy, with no definite advantage of surgery.
Interest in imaging of carotid atheroma is now shifting from focusing on luminal stenosis to identification of the vulnerable plaque. This shift is a result of the greater understanding of the pathogenesis of the disease and the acknowledgment that strokes can still occur in populations not considered suitable for surgical management in the previously mentioned studies (eg, 30%–49% stenosis). Also, some patients identified as appropriate surgical candidates using the criteria of degree of stenosis may have clinically/pathologically stable disease. This concept of a vulnerable plaque is not new, with the phrase having been originally introduced by Muller and colleagues in 1985 in relation to studies of coronary artery disease. This concept has been applied to other vascular territories affected with atherosclerosis, but it is only recently that noninvasive imaging of plaque has become possible, and the carotid artery is far more amenable to this than the coronary artery.
Luminal imaging
Although digital subtraction angiography (DSA) is still the gold standard for assessing the degree of stenosis in carotid atherosclerosis, because of the risks associated with an interventional procedure, most patients are now investigated and managed based on noninvasive methods that have compared well with DSA.
Ultrasound
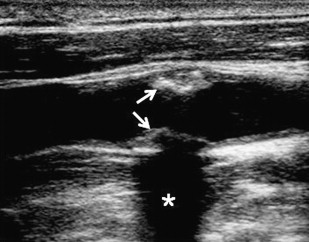
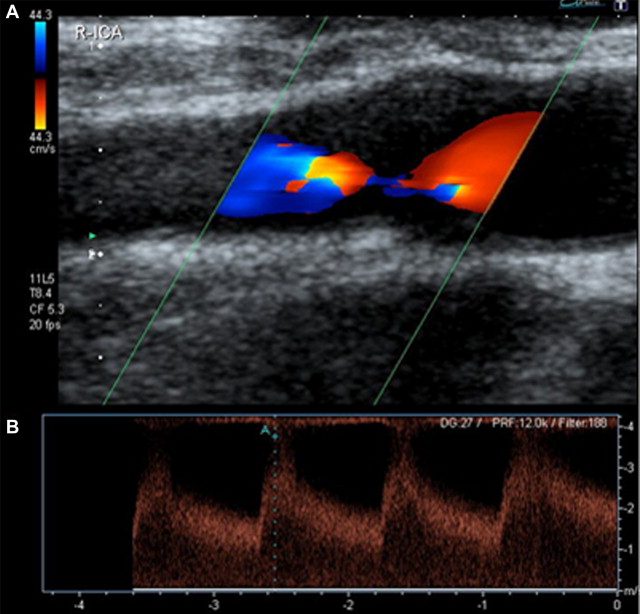
The most widely available and used method for assessing carotid disease is ultrasound (US). It is an established and inexpensive technique with proven accuracy. Two methods of US are commonly used for assessment of carotid disease (B-mode and Doppler ultrasound [DUS]), and these are often combined. B-mode US allows visualization of the lumen and the vessel wall so that luminal diameters may be assessed ( Fig. 1 ). DUS relies on measurement of blood velocity in the vessel to calculate the degree of stenosis ( Fig. 2 ). Although studies have shown the accuracy of DUS, there are limitations to the technique: it is operator dependent, subject to artifacts in calcified disease (see Fig. 1 ), and inaccurate in distinguishing near occlusion. However, even with these limitations, sensitivities and specificities compare well with the other imaging modalities. It is particularly useful as an exclusionary examination before proceeding onto more costly imaging modalities.
Computed Tomography Angiography
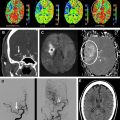
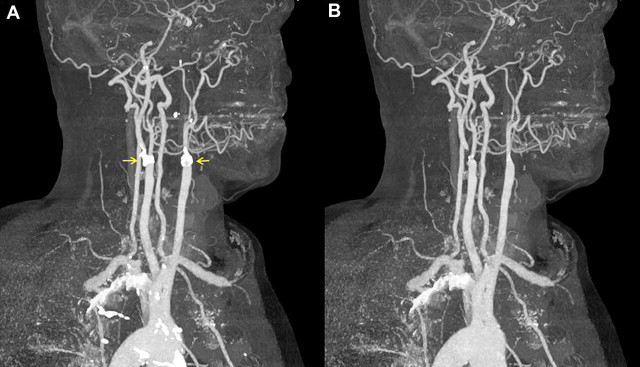
Computed tomography angiography (CTA) is a popular noninvasive method of carotid imaging. It allows for rapid imaging and a large anatomic coverage, from the arch of the aorta to the circle of Willis. It is also minimally invasive, requiring only peripheral venous injection of iodinated contrast media. Axial source images can be postprocessed in several ways (eg, maximal intensity projections [MIPs], multiplanar reformatting) to produce a three-dimensional (3D) angiogram. CTA is well tolerated as an investigation by patients, but there are some disadvantages. CTA involves ionizing radiation, which means it is less desirable for repeated/follow-up examinations. CTA is highly specific, but is arguably the least sensitive of the noninvasive methods for assessing luminal stenosis compared with DSA ; however, comparisons have shown a good level of agreement with DUS. Another issue can be the method of measurement. For example, when stenotic measurements taken as diameters from DSA were compared with area measurements from CTA, CTA tended to underestimate the degree of stenosis. There can also be issues with heavy calcification where assessment of the degree of stenosis is obscured by the calcification, although image processing methods may alleviate this problem ( Fig. 3 ). CTA can also be used to detect other plaque features such as ulceration, which is evidence of vulnerable disease.
Magnetic Resonance Angiography
Contrast-enhanced magnetic resonance angiography (CE-MRA) is gaining in popularity because of the high sensitivity and specificity that has been shown compared with DSA; it is arguably the best of the noninvasive modalities, and the multimodal approach of DUS and CE-MRA enhances the already high sensitivity/specificity. Combined with this good diagnostic performance, the cost-effectiveness compared with DSA makes it an attractive imaging option. However, in some centers, because magnetic resonance (MR) is not as accessible as US or CTA, it is not the imaging method of choice. Several different methods have been used in different clinical trials for measuring degree of stenosis. When comparing these methods for CE-MRA and DSA, the NASCET criteria performed most consistently between the 2 examinations and therefore is the most appropriate method of assessing CE-MRA images. Like CTA, this is a minimally invasive technique that produces high-quality images with a large anatomic coverage ( Fig. 4 ), with the added advantage of no ionizing radiation.
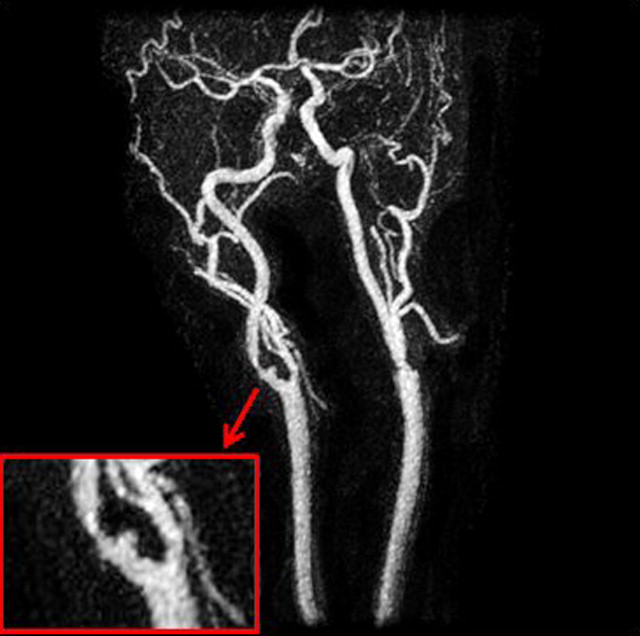
Unenhanced MRA can also be performed, usually as time-of flight (TOF) imaging, which can be either a two-dimensional (2D) slice or 3D volumetric acquisition. Both acquisitions can be viewed in a slice or a MIP format. Although this method has the benefit of no contrast administration, there are issues with the diagnostic quality of this technique. In general, unenhanced TOF MRA has shown better diagnostic accuracy than CTA compared with DSA but has a tendency to overestimate the degree of stenosis. Furthermore, it cannot reliably distinguish near occlusion from occlusion when there is no flow-related enhancement within the internal carotid artery. In addition, T1 hyperintense thrombus can give the appearance of luminal patency, although on 3D TOF imaging it has been proposed that the halo sign may indicate the presence of wall thrombus. Flow artifacts can also affect the diagnostic quality of the images.
Plaque imaging
As the understanding of atherosclerosis as a disease process has evolved, the focus has moved from simple measurement of stenosis to considering the importance of plaque composition, biologic markers, and biomechanical properties. These factors are key to the concept of vulnerable plaque versus stable disease and are all believed to be linked to developing disease and eventual translation from subclinical to symptomatic disease.
Morphologic Imaging
Pathologic studies have shown that atheromatous disease can undergo outward remodeling that can lead to a significant disease burden being present with limited luminal compromise. Other features that have been identified as making a plaque more vulnerable are a large, lipid-rich necrotic core; a thin or ruptured fibrous cap; plaque ulceration; and the presence of intraplaque hemorrhage. In the coronary artery, the presence of calcified nodules is an accepted criterion for vulnerable disease. In contrast, in the carotid artery, it is not the presence of calcification that is believed to affect plaque but its location within the plaque, with calcium deposits within a thin fibrous cap increasing maximal plaque wall stresses compared with deposits at other locations that have little effect.
US
US has been used in 3 different ways to try to assess plaque vulnerability: assessment of plaque echogenicity, measurement of intima media thickness (IMT), and contrast-enhanced US. Contrast-enhanced US is used to assess pathologic processes and is discussed later.
Carotid plaque echolucency on B-mode US, using a method called median gray scale (GSM), has been related to both clinically symptomatic disease and the presence of cerebral infarction on CT. Comparison with histologic specimens has shown that plaques that have a low GSM on US histologically have high-risk features such as high lipid and hemorrhage content. Echogenicity seems to be a risk factor for symptoms independently of the degree of stenosis present and seems to be an indicator of risk in individuals who have experienced silent nonlacunar cerebral infarcts. Low GSM (<25) has also been linked to increased risk of stroke with carotid stenting procedures. IMT, an early measure of carotid atherosclerotic disease, has been correlated with the presence of plaque, but the direct link to stroke/TIA is not as clear. However, recent work in patients with coronary disease undergoing carotid assessment has indicated that the combination of both IMT and echogenicity of the internal carotid artery could be of value in prediction of coronary risk. Furthermore, IMT in the common carotid artery and Framingham Risk Score independently predicted carotid artery atherosclerotic plaque formation at 5 years, in initially disease-free internal, external, or common carotid arteries, in a population-based longitudinal study of 1922 patients.
High-resolution magnetic resonance imaging
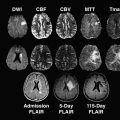
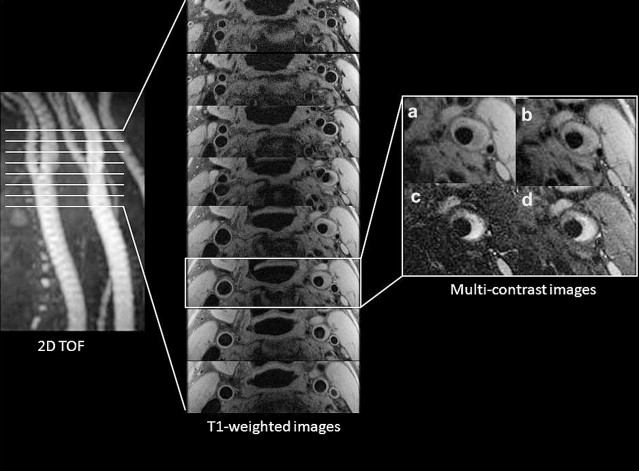
High-resolution magnetic resonance imaging (MRI) has been used to show plaque features with favorable comparison with histology. Angiographic TOF images are acquired to localize the disease-affected section of the vessel, then multicontrast imaging (T1-weighted, T2-weighted, and proton density–weighted images) are acquired through this segment at matched slice locations ( Fig. 5 ). Short tau inversion recovery (STIR) images can also provide a benefit in plaque characterization by showing a distinct signal difference between fibrous cap and lipid core. Review of these images then allows the plaque morphology to be identified ( Table 1 ), and a modified version of the American Heart Association (AHA) plaque classification has been created for MRI. Morphologic imaging has allowed plaque characteristics to be assessed in mild (<50% stenosis) to moderate (50%–69% stenosis) disease, for which previously there was little information because surgical intervention is not offered. At this earlier stage of disease, hemorrhage and larger lipid core have been independently shown to be associated with thin/ruptured fibrous caps, all markers of vulnerable disease. The rate of vulnerable lesions (AHA type VI) at mild levels of stenosis seems to be much higher than was generally appreciated (29.8% prevalence in arteries with a stenosis of 49% or less, increasing with degree of stenosis). The advantages of this method of MRI have been exploited to perform longitudinal assessments of disease progression and to assess evolution of disease characteristics such as hemorrhage, as well as to monitor responses to pharmacologic intervention. Multiple features of vulnerability, as detected by high-resolution imaging, have been linked to symptoms, compared with asymptomatic counterparts: presence of thrombus, large lipid core, thin/ruptured fibrous cap, and type VI lesions.
| T1-Weighted | T2-Weighted | Proton Density–Weighted | STIR | Contrast-enhanced T1-Weighted | ||
|---|---|---|---|---|---|---|
| Lipid-rich Necrotic Core | — | —/↑ | —/↓ | —/↑ | ↓ | ↓ |
| Fibrous Tissue | — | —/↑ | — | — | ↑ | ↑ |
| Hemorrhage | Fresh | ↑ | ↓ | ↓ | ↓ | — |
| Recent | ↑ | ↑ | ↑ | ↑ | — | |
| Calcification | — | ↓ | ↓ | ↓ | ↓ | ↓ |
Addition of other sequences can aid plaque characterization. One sequence proposed to aid in the diagnosis of vulnerable plaque is a gradient echo sequence termed direct thrombus imaging, which has been used to show high signal in symptomatic disease compared with contralateral asymptomatic vessels, suggesting that it may be a method of identifying vulnerable plaque. Diffusion-weighted imaging (DWI) is another method that has been proposed to aid the delineation of plaque components in unenhanced imaging. Ex vivo imaging indicated that DWI provided the best method for separating lipid from other tissue types. The first in vivo carotid study has shown promising results for using apparent diffusion coefficient (ADC) values derived from DWI for distinguishing lipid (lower ADC) and fibrous tissue.
The addition of gadolinium contrast media greatly enhances the differentiation of fibrous tissue (more enhancement) and lipid on morphologic MRI. By using this technique, one study found that the incidence of vulnerable plaque was significantly higher in nonoperable disease (by stenosis criteria) than in the severely stenotic lesions. One of the issues that has kept plaque MRI in the research rather than clinical setting has been the lengthy imaging times. Comparison of the number of sequences needed for classification found that there was good agreement between plaque classification using 3 sequences (T1-weighted, contrast-enhanced T1-weighted, and TOF) and 5 sequences (T1-weighted, contrast-enhanced T1-weighted, T2-weighted, proton density–weighted, TOF), which would reduce the imaging time.
Imaging of Pathologic Processes
Further risk stratification for future clinical sequelae from carotid atherosclerosis can be made by imaging the pathophysiology of the disease. Two disease processes with the potential to be imaged are plaque neovascularization and inflammation. Initially lipoproteins aggregate in the vessel wall and become oxidized, triggering the production of endothelial molecules and activation of endothelial receptors, causing monocytes to be recruited into the vessel wall. The monocytes develop into activated macrophages that ingest the lipoproteins and then transform into foam cells, creating the lipid-rich necrotic core. This process provides the inflammatory component of atherosclerosis. As the plaque expands, the oxygen demand increases, leading to the formation of neovessels from the vasa vasorum extending into the plaque. This process has been linked to inflammation and may be a route by which inflammatory cells enter.
Neovascularization
Both MRI and contrast-enhanced ultrasound (CEUS) have been used to image this process. CEUS uses gas-filled microbubbles injected intravenously, which have a different echogenicity to soft tissue. Their general structure consists of a hydrophilic microbubble shell (commonly albumin or lipid) with an echogenic gas core such as perfluorocarbon or nitrogen.
Several studies have been conducted in the past few years using CEUS to examine plaque neovascularization. Comparison of imaging with histologic specimens from subjects who underwent carotid endarterectomy showed that those plaques with enhancement had a significantly higher vasa vasorum density than those without an enhancement pattern; the plaques that enhanced the most were also more echolucent. It has been suggested that CEUS could be used as a method for plaque risk stratification because it has been found that symptomatic plaques enhanced significantly more than asymptomatic plaques (n = 104, 35 symptomatic).
MRI using gadolinium chelates provides information on plaque neovascularization in addition to plaque composition and luminal information. MRI can be used to image neovascularization in 1 of 2 ways: delayed or dynamic imaging. Areas of plaque that strongly enhance on delayed T1-weighted imaging have a good correlation with the histologic presence of neovascularization.
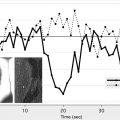
The feasibility of dynamic contrast-enhanced MRI (DCE-MRI) for human carotid imaging was first proposed in 1999 and later pursued further, imaging with dedicated phased array coils, using a spoiled gradient echo sequence without cardiac gating, giving a temporal resolution of 15 seconds. Fractional blood volume was used to assess the images, and measurements were higher in the areas that correlated to the presence of microvessels on histology. Later work used K Trans and Vp as metrics of neovascularization derived from the DCE-MR. These metrics are widely used in the main application of DCE, namely tumors. K Trans and Vp measurements correlated not only with the presence of neovascularization on histology but also macrophages, suggesting a link between neovascularization and inflammation. K Trans was also found to correlate with clinical parameters, with measurements being higher in smokers than nonsmokers, and K Trans being significantly higher in patients requiring carotid endarterectomy than in those with moderate stenosis. Several different gadolinium agents are available and comparison of 2 indicated that, although measurements are similar, K Trans values do differ between agents, and this should be considered when comparing results.
Inflammation
Three different methods have been used to image inflammation: CEUS, positron emission tomography (PET), and MRI. The microbubbles used for CEUS are phagocytosed by monocytes but remain acoustically active for up to 30 minutes. Therefore, late-phase US imaging has been used to assess whether microbubbles can potentially detect inflammation in vivo. Greater enhancement was seen in symptomatic compared with asymptomatic plaques but no histology was available to determine whether inflammation was present.
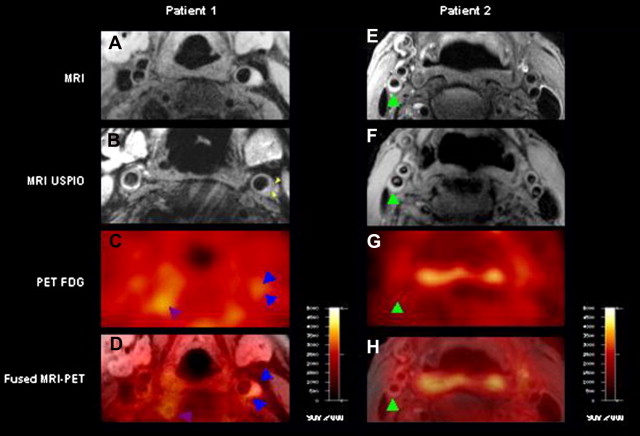
PET using 18 F-fluorodeoxyglucose (FDG) has been widely used for showing inflammation. It uses radiolabeled glucose to highlight areas of increased glucose consumption, such as inflammation. However, this is nonspecific because it shows all areas of increased glucose uptake. The other disadvantage of PET imaging is that it has a low resolution; so images need to be coregistered to CT or MRI to accurately identify the anatomic site of tracer uptake. However, using combined FDG-PET/CT imaging has shown good detection of vascular inflammation compared with histology, with good reproducibility. It has also shown that symptomatic lesions had a higher level of inflammation compared with contralateral asymptomatic disease (n = 6). FDG-PET of recently symptomatic patients (TIA), examining target lesions identified at angiography, found that the suspicious lesion identified at angiography may not always be the lesion that causes symptoms. For 3/12 cases, the target lesions had low uptake, but other nonstenotic lesions in the appropriate territory for the symptoms had high uptake, suggesting that these vulnerable plaques were more likely causative lesions. PET/CT combined with multicontrast MRI has correlated the findings of inflammatory change with lipid burden within carotid plaque, a previously mentioned vulnerable feature.
MRI for inflammation uses ultrasmall paramagnetic iron oxides (USPIOs). USPIOs have been used both in animal and human studies to show the presence of macrophages, and thereby inflammation, within carotid plaque. USPIOs are nanoparticles (typically approximately 50 nm in diameter, containing iron oxide particles within a dextran or siloxane coat) that become incorporated within the macrophages that are then taken up into the inflammatory atherosclerotic plaque. To allow time for this process and maximize the uptake within the plaque, imaging after contrast administration is delayed to an optimal time window; one study showed this to be 24 to 48 hours in human carotid MRI for ferumoxtran-10. On postcontrast imaging, USPIOs act as a negative contrast medium by decreasing T2 relaxation and creating areas of signal loss on T2- and T2*-weighted imaging.
Examination of plaque inflammation, as shown by USPIO uptake, and degree of luminal stenosis found no correlation between the 2 measurements, suggesting that they are likely to be independent risk factors for stroke. From a morphologic point of view, USPIO uptake was detected in most (27/36) of the plaques that had ruptured or were rupture prone, but in only 1 of the 14 plaques that had a stable morphologic configuration, suggesting a relationship between inflammation and vulnerability. In a comparison of symptomatic and asymptomatic disease, USPIO uptake was significantly higher in symptomatic plaques, suggesting that symptomatic disease was more inflammatory and asymptomatic disease more stable. However, inflammation was still seen in the contralateral, asymptomatic side of individuals who had experienced carotid-related symptoms even though generally the inflammation was to a lesser extent and the stenosis was to a lower degree, similar to the findings of the PET studies. Comparison of contralateral asymptomatic disease, in symptomatic individuals, with truly asymptomatic disease, found that even truly asymptomatic disease showed inflammation, although to a lesser extent than other disease, but this evidence suggests that there may be a case for monitoring and treating asymptomatic disease.
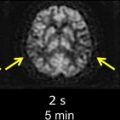
The use of USPIO imaging has taken the application of carotid imaging a stage further by monitoring the short-term response to therapy in the ATHEROMA study. Forty-seven patients with carotid stenosis underwent USPIO-enhanced MRI and were randomized to high- or low-dose atorvastatin (80 or 10 mg). A significant reduction in plaque inflammation, as shown by signal loss with USPIO, was seen at 12 weeks, showing that this type of imaging can be used to assess response to therapy in a short time period ( Fig. 6 ).
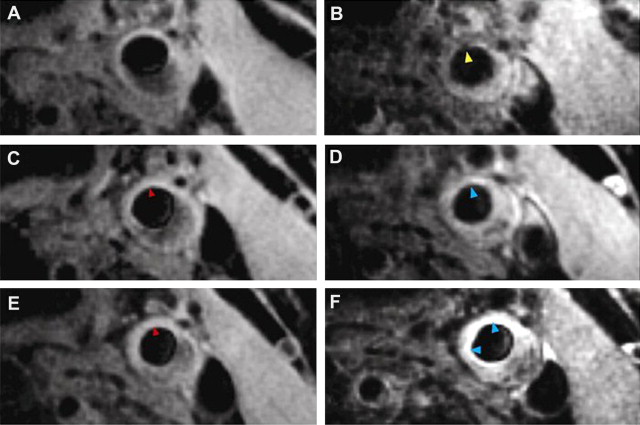
PET and MRI use different methodologies but a good concordance has been shown between the two modalities for detecting inflammation ( Fig. 7 ). Both PET and USPIO imaging have shown that inflammatory disease is associated with a higher microemboli count to the brain (a surrogate marker of cerebrovascular disease), as detected by transcranial DUS compared with noninflammatory disease, indicating that inflammatory disease may be associated with a higher risk of future events. The administration of high-dose, short-term (12 weeks) statin therapy reduced the microemboli counts in inflammatory disease, as detected by USPIO on MRI, compared with baseline counts, suggesting that statin therapy may, even in such a short time, not only reduce plaque inflammation, but also lead to some degree of plaque stabilization.
Biomechanics and Imaging
An application of imaging that is becoming more widely accepted in the assessment of vulnerable plaque is biomechanical modeling of plaque stresses. The opportunity to apply this idea in vivo to individuals arose with the information provided by MRI. Two types of stresses have been proposed as being important in atherosclerosis: mechanical stress and wall shear stress, both of which can be derived from MR imaging. Although it has been suggested that shear stress may have a role in initiation of atherosclerosis and plaque development, modeling-based MRI of plaque morphology and flow indicates that mechanical stress is more likely to play a role in plaque rupture. Stresses on the fibrous caps of ruptured plaques (defined on MRI) are higher than those on the fibrous caps of unruptured plaques. The location of calcification within the plaque also affects the stresses, with calcification that is within the fibrous cap causing the maximal plaque stress. Furthermore, MRI-based modeling has shown that maximal predicted plaque stresses in symptomatic patients are higher than those in asymptomatic patients, suggesting that plaques with higher stresses may be more rupture prone. Inflammation may also add to this complex picture, leading to plaque rupture; imaging with USPIO of carotid atheroma combined with biomechanical modeling has shown that maximal stresses are congruent with sites of inflammation in the plaque. Furthermore, biomechanical stress simulations have been used to study plaque evolution in patients with TIA; fresh plaque hemorrhage was associated with 30% higher maximum critical stress than chronic plaque hemorrhage in one study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree