With more than 700,000 strokes per year resulting in greater than 160,000 deaths per year, stroke remains the leading cause of disability and third leading cause of death in the United States. Despite an overall decline in stroke mortality over the past 40 years, the total number of stroke deaths continues to increase, suggesting an increase in stroke incidence. The last 20 years of neuroscience advances have moved stroke from a condition that is monitored clinically and imaged serially as it evolves to an entity that can be treated acutely, with remarkable alterations in its natural history.
With more than 700,000 strokes per year resulting in greater than 160,000 deaths per year, stroke remains the leading cause of disability and third leading cause of death in the United States. Despite an overall decline in stroke mortality over the past 40 years, the total number of stroke deaths continues to increase, suggesting an increase in stroke incidence. The last 20 years of neuroscience advances have moved stroke from a condition that is monitored clinically and imaged serially as it evolves to an entity that can be treated acutely with remarkable alterations in its natural history.
Imaging triage
Approval from the US Food and Drug Administration (FDA) for the intravenous (IV) administration of the thrombolytic recombinant tissue plasminogen activator (rt-PA) in the treatment of acute ischemic stroke (AIS) requires exclusion of intracranial hemorrhage (ICH) by imaging. Although nonenhanced computed tomography (NECT) is a widely available modality used to exclude ICH in the treatment of AIS, the FDA approval recommends exclusion of ICH by “CT or other diagnostic imaging method similarly sensitive for the presence of hemorrhage.” Similarly, the American Heart Association (AHA) Scientific Statement Guidelines for the Early Management of Adults with Ischemic Stroke gives no single recommendation as to what imaging modality should be used. The AHA Scientific Statement Recommendations for Imaging of Acute Ischemic Stroke recommends the use of NECT or magnetic resonance (MR) imaging for evaluation of ICH and frank ischemic changes. Furthermore, in patients presenting within 3 hours, the Guidelines state, “there is a suboptimal detection rate of ischemic changes with NECT alone, and a more definitive diagnosis will be obtained with magnetic resonance – diffusion weighted images (MR-DWI) or CT Angiographic-source images (CTA-SI) … if this does not unduly delay the timely administration of tPA.” In addition, a vascular study (CTA, MR angiography, digital subtraction angiography [DSA]) is “probably indicated.” For patients beyond 3 hours, MR–diffusion-weighted imaging [DWI] or CTA-SI “should be performed along with vascular imaging and perfusion studies, particularly if mechanical thrombectomy or intra-arterial (IA) thrombolytics therapy is contemplated.”
Nonenhanced CT has been used as the sole imaging study in the initial evaluation of patients for AIS treatment in multiple landmark trials because it has a historical ability to exclude ICH. MR, specifically gradient-echo sequences, is at least as accurate as NECT in the detection of ICH in patients with acute stroke and has a 96% concordance with NECT, although an advantage of NECT is seen when evaluating for subarachnoid hemorrhage. In the National Institute of Neurologic Disorders and Stroke (NINDS) rt-PA Stroke Study, NECT showed 31% specificity for signs of early infarction, which is significantly low given an average National Institute of Health Stroke Scale (NIHSS) score of 14. MR-DWI is clearly the most accurate modality and sequence in the evaluation of AIS, with a significant improvement in sensitivity and specificity in the evaluation of stroke when compared with CT (at 6 hours DWI sensitivity/specificity 91%/95% vs NECT 61%/65%).
NECT has been shown to be of diagnostic and prognostic value when a hyperdense middle cerebral artery sign (HMCAS) is present. Both the presence of a hyperdense middle cerebral artery (MCA) and the expected concurrent high NIHSS score are predictors of poor outcomes in acute stroke treatment. The results of a retrospective analysis suggest that intra-arterial therapy (IAT) is more beneficial than IV thrombolytic therapy in patients with HMCAS on NECT images. HMCAS was observed in 55 patients treated with IAT (n = 268) and 57 patients treated with IV therapy (n = 249). The maximum delay time from symptom onset to IV therapy and IAT was 3 hours and 6 hours, respectively. There was no difference between the 2 groups in terms of baseline stroke severity and patient age. Mean time to treatment was longer in the IAT group (244 ± 63 minutes) than in the IV therapy group (156 ± 21 minutes; P = .0001). A favorable outcome (modified Rankin scale [mRS score] ≤2) was more frequently observed after IAT (n = 29, 53%) than after IV therapy (n = 13, 23%; P = .001). The mortality was also lower in the IAT (n = 4, 7%) than in the IV therapy group (n = 13, 23%; P = .022). Results of multiple regression analysis showed that IAT was associated with a more favorable outcome but similar mortality to IV therapy.
Additional triage with vascular and perfusion imaging has many goals. Normal imaging suggests a resolved vascular or nonvascular cause (such as seizure) for presenting symptoms. In the positive imaging examinations, advanced vascular and perfusion imaging helps select for 2 diverging populations. One is the patient with clear large-vessel occlusion and large infarct (>approximately 100 mL). This population has a poor neurologic outcome regardless of whether IV therapy or IAT results in vessel recanalization. A second group is one that displays vascular stenosis or occlusion with ischemic but not infarcted (or not completely infarcted) tissues that would benefit from endovascular intervention.
MR imaging in the evaluation and triage of patients with AIS has been criticized for limited access and long study time; however, it has been shown that MR imaging can be used rapidly and efficiently, resulting in improved patient evaluation.
Ultrafast MR imaging evaluation of AIS consisting of axial T1, T2, fluid-attenuated inversion recovery, DWI, and contrast-enhanced T1 imaging can be performed and interpreted in 15 minutes or less (as defined by the interval between the patient leaving the emergency department and returning). Review of a group of 97 patients with AIS being evaluated for acute intervention was performed by the same group. After clinical evaluation and NECT was performed, preliminary clinical diagnosis (transient ischemic attack [TIA], small vessel ischemic disease, large-vessel ischemic disease) and prescribed treatment (no treatment, IAT, IV therapy, IV therapy/IAT) were tabulated, the patient was sent for ultrafast MR imaging. Twenty-six percent (25/97) of patients had therapy changed from presumptive plan after CT to definitive plan after MR imaging. Of these patients 52% (13/25) had therapy changed from thrombolytic to nonthrombolytic treatment, 20% (5/25) had therapy changed from planned intra-arterial (IA) intervention to IV rt-PA because of perfusion abnormality confined to the basal ganglia and not the suspected large-vessel occlusion, 4% (1/25) had therapy changed from IV rt-PA to IA intervention, and 12% (3/25) of patients had therapy changed from no treatment to IA intervention.
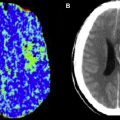
The usefulness of CT perfusion (CTP) in predicting the final infarct volume and clinical outcomes in patients with acute MCA infarction after intra-arterial thrombolysis has been investigated. Results of a retrospective study involving 22 patients with acute MCA occlusion who had IA thrombolysis showed that baseline CTP (CTA source image) lesion volumes are significantly correlated with the final infarct volume ( P = .0002) and clinical outcome ( P = .01). The relationship between the baseline and the final volumes was stronger for those patients who had successful recanalization. A progression of the infarct lesion volume was observed in patients without recanalization. All patients (100%) with a baseline perfusion lesion volume more than 100 mL and without recanalization had a poor outcome (mRS score >3). Conversely, most of the patients (77%) with baseline perfusion lesion volume less than 100 mL and complete recanalization had good or fair functional outcome (mRS score ≤3).
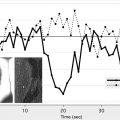
Of great interest to the interventionalist is the difference between the window of intervention based on the absolute time of onset and the physiologic time window. In a retrospective study, the outcomes of IAT during an 8- to 24-hour window in 30 patients with stroke (premorbid mRS score 1; mean NIHSS score 13, range 5–22) identified using CTP imaging were reported. IAT was performed only in those patients who had clinically significant salvageable brain tissue (>30% relative cerebral blood volume values) compared with established core infarct (< one-third of the MCA territory). Patients with established infarct core in larger regions than one-third of the MCA territory were not offered endovascular therapy. IAT was performed using intra-arterial thrombolysis (n = 10), mechanical thrombectomy (n = 21), and balloon angioplasty (n = 14); some patients had a combination of treatments. Successful recanalization (Thrombolysis in Myocardial Infarction [TIMI] score 2/3) and symptomatic ICH were observed in 20 (66.7%) and 3 (10%) of the patients, respectively. Seven (23.3%) patients died during hospitalization as a result of procedural complications, disease progression, or associated morbidities. Mean NIHSS score at discharge was 9.5, representing an overall NIHSS 3.5-point improvement from the baseline values. A total of 10 (33.3%) patients died within 3 months of IAT. The mean mRS score of the survivors after 3 months was 3. The results showed the feasibility and potential usefulness of IAT in a select group of patients with stroke with salvageable brain tissue as identified from CTP images well outside the traditional time from onset ( Fig. 1 ).
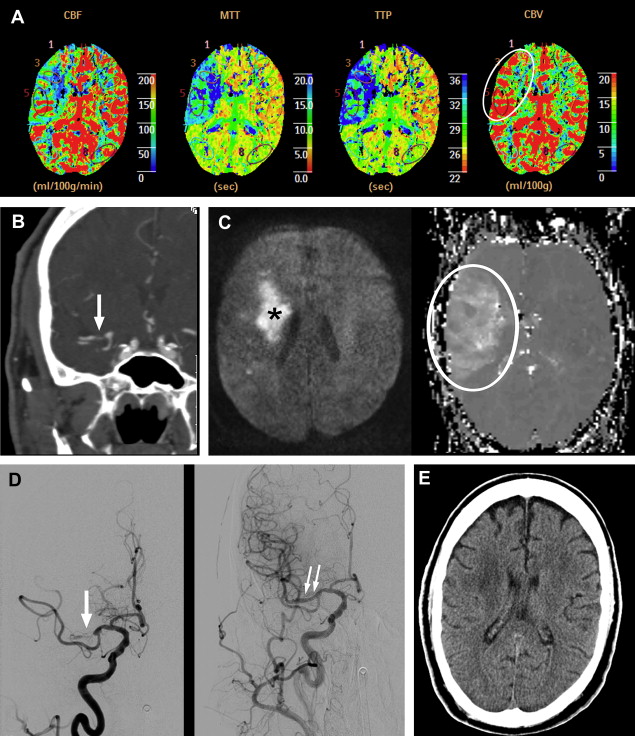
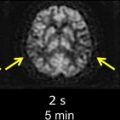
An application of clinical-diffusion mismatch for triage of patients with AIS has also been studied using MR imaging. In a case series of 11 patients with NIHSS score 8 or greater and limited abnormality on the diffusion-weighted images (smaller than 25 cm) beyond 8 hours of symptom onset, endovascular therapy was performed. All the patients underwent reperfusion therapy after between 12 and 24 hours of symptom onset. A successful recanalization was achieved in 8 patients. The mean NIHSS scores at baseline, 24 hours, and 1 week were 14 ± 4, 11 ± 7 and 6 ± 5, respectively. The mean NIHSS score of those patients who had successful recanalization was 8 ± 5 at 24 hours and 4 ± 3 at 1 week. An improvement of 4 points or greater in NIHSS score was observed in all 8 patients who had successful recanalization. None of the patients in this study had an ICH or worsening of the NIHSS score by 4 points or greater (see Fig. 1 ).
In a comparison study of CTP and MR perfusion (MRP) examining core/penumbra mismatch, 47 patients presenting with acute anterior circulation stroke within 9 hours of onset received both CTP and MRP studies within 3 hours of each other; they were evaluated for criteria used in trial selection: core infarct less than 100 mL, mean transit time (MTT) lesion greater than 2 cm in diameter, and greater than 20% DWI/MTT mismatch. When excluding for lesions that were inadequately visualized on CTP (because of the limited brain coverage that exists with some CTP packages), the correlation of core/penumbra imaging between CTP and MRP for trial selection showed overall agreement of 97.8%.
As the role of IA intervention expands in AIS so will the role of triage imaging. Other than the need to exclude ICH, the role and modality of imaging has not been uniformly defined. This situation will not likely change in the near future because issues such as hardware availability and operator experience and preference will greatly influence what is done at individual centers.
IAT
Chemical Thrombolysis
There is no drug approved by the FDA for IA chemical thrombolysis. The only FDA-approved drug in the treatment of AIS is the IV administration of rt-PA alteplase. Approved in 1996, its indication is to treat acute stroke within 3 hours of onset of ischemic symptoms.
The Phase II Prolyse in Acute Cerebral Thromboembolism (PROACT) trial was the first randomized, double-blind trial to test the safety and recanalization efficacy of IA-targeted delivery of the plasminogen activator agent recombinant prourokinase (r-proUK) versus saline placebo. Inclusion criteria included randomization and treatment within 6 hours of symptom onset, minimum NIHSS score of 4 (with exception made for aphasia or isolated hemianopsia) and age 18 to 85 years. A total of 105 patients progressed to angiography of 1314 patients screened. Forty-six patients were randomized, and after 6 late exclusions, 40 patients with documented M1 or M2 MCA occlusions (TIMI score 0 or 1) were treated. With a 2:1 treatment/placebo protocol, 26 patients received r-proUK 6 mg IA within the thrombus and 14 received placebo of saline IA. Initially, patients received IV heparin after angiographic verification of thrombus. This high-heparin protocol received by the first 16 patients consisted of a 100-IU/kg bolus followed by continuous infusion of 1000 IU/h for 4 hours. After early analysis of these first 16 patients, 8/11 (73%) of those patients treated with r-proUK showed ICH within 24 hours, and a protocol change consisting of a low-heparin regimen was initiated. This low-heparin protocol gave both treatment arms a heparin bolus of 2000 IU IV and a subsequent drip at 500 IU/h for the following 4 hours, although at the price of increased rates of ICH, the concurrent administration of higher-dose IV heparin in PROACT led to increased recanalization rates. Analysis of both heparin regimens disclosed an overall 42% versus 7% ICH rate favoring placebo, with a reduction to a 15% rate for symptomatic ICH in the combined r-proUK group and no change in the placebo group. Overall recanalization (defined as TIMI score 2 or 3) was 58% for the r-proUK group and 14% for the placebo group. Most importantly, 90-day mortality was reduced from 43% in the placebo group to 27% in the r-proUK arm, and a 10% to 12% absolute increase in excellent neurologic outcome as defined by normal or near normal 90-day functional assessments and NIHSS score.
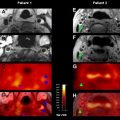
The use of IA chemical thrombolysis is based largely on the results of the PROACT II trial ( Fig. 2 ). PROACT II was a randomized, controlled trial in which 180 patients (12,323 patients screened) experiencing new, focal neurologic signs in the MCA distribution were randomized to either treatment or control with similar PROACT inclusion criteria. Treatment consisted of the administration of r-proUK 9 mg IA at the site of thrombus over 2 hours. The control patients received no IAT. Both arms received the low-heparin protocol initiated in the PROACT trial. Results favored IA treatment with improved recanalization rates (TIMI 2 or 3 flow: 66% treatment, 18% control) and clinical outcome (mRS score 2 or less: 40% treatment, 25% control) but with no significant difference in mortality, which was approximately 25% at 90 days for both groups. In addition, the r-proUK 9 mg IA dose showed improved outcomes compared with the PROACT r-proUK 6 mg dose despite a 4% increase in symptomatic ICH (SICH) when comparing r-proUK-treated PROACT II patients with the low-heparin population in PROACT. The results of PROACT II prompted the AHA Stroke Council to change IA thrombolysis from experimental treatment to an accepted treatment of AIS. The use of r-proUK was widely used until its withdrawal from the US market in 1999.
IAT
Chemical Thrombolysis
There is no drug approved by the FDA for IA chemical thrombolysis. The only FDA-approved drug in the treatment of AIS is the IV administration of rt-PA alteplase. Approved in 1996, its indication is to treat acute stroke within 3 hours of onset of ischemic symptoms.
The Phase II Prolyse in Acute Cerebral Thromboembolism (PROACT) trial was the first randomized, double-blind trial to test the safety and recanalization efficacy of IA-targeted delivery of the plasminogen activator agent recombinant prourokinase (r-proUK) versus saline placebo. Inclusion criteria included randomization and treatment within 6 hours of symptom onset, minimum NIHSS score of 4 (with exception made for aphasia or isolated hemianopsia) and age 18 to 85 years. A total of 105 patients progressed to angiography of 1314 patients screened. Forty-six patients were randomized, and after 6 late exclusions, 40 patients with documented M1 or M2 MCA occlusions (TIMI score 0 or 1) were treated. With a 2:1 treatment/placebo protocol, 26 patients received r-proUK 6 mg IA within the thrombus and 14 received placebo of saline IA. Initially, patients received IV heparin after angiographic verification of thrombus. This high-heparin protocol received by the first 16 patients consisted of a 100-IU/kg bolus followed by continuous infusion of 1000 IU/h for 4 hours. After early analysis of these first 16 patients, 8/11 (73%) of those patients treated with r-proUK showed ICH within 24 hours, and a protocol change consisting of a low-heparin regimen was initiated. This low-heparin protocol gave both treatment arms a heparin bolus of 2000 IU IV and a subsequent drip at 500 IU/h for the following 4 hours, although at the price of increased rates of ICH, the concurrent administration of higher-dose IV heparin in PROACT led to increased recanalization rates. Analysis of both heparin regimens disclosed an overall 42% versus 7% ICH rate favoring placebo, with a reduction to a 15% rate for symptomatic ICH in the combined r-proUK group and no change in the placebo group. Overall recanalization (defined as TIMI score 2 or 3) was 58% for the r-proUK group and 14% for the placebo group. Most importantly, 90-day mortality was reduced from 43% in the placebo group to 27% in the r-proUK arm, and a 10% to 12% absolute increase in excellent neurologic outcome as defined by normal or near normal 90-day functional assessments and NIHSS score.
The use of IA chemical thrombolysis is based largely on the results of the PROACT II trial ( Fig. 2 ). PROACT II was a randomized, controlled trial in which 180 patients (12,323 patients screened) experiencing new, focal neurologic signs in the MCA distribution were randomized to either treatment or control with similar PROACT inclusion criteria. Treatment consisted of the administration of r-proUK 9 mg IA at the site of thrombus over 2 hours. The control patients received no IAT. Both arms received the low-heparin protocol initiated in the PROACT trial. Results favored IA treatment with improved recanalization rates (TIMI 2 or 3 flow: 66% treatment, 18% control) and clinical outcome (mRS score 2 or less: 40% treatment, 25% control) but with no significant difference in mortality, which was approximately 25% at 90 days for both groups. In addition, the r-proUK 9 mg IA dose showed improved outcomes compared with the PROACT r-proUK 6 mg dose despite a 4% increase in symptomatic ICH (SICH) when comparing r-proUK-treated PROACT II patients with the low-heparin population in PROACT. The results of PROACT II prompted the AHA Stroke Council to change IA thrombolysis from experimental treatment to an accepted treatment of AIS. The use of r-proUK was widely used until its withdrawal from the US market in 1999.