Chapter Outline
Types of Peritoneal Fluid Collections
Pathways of Fluid Distribution
Ascites is the pathologic accumulation of fluid in the peritoneal cavity. It is a common clinical finding that can be associated with a large number of diseases. In some disorders, peritoneal fluid represents a complication or late manifestation of disease, whereas in others, it is the first clinical expression of the disease process. For this reason, early detection and characterization of ascites and other peritoneal fluid collections are important.
Pathophysiology
The causes of ascites are protean and are listed in Box 110-1 . The imaging features that can help differentiate the various causes are listed in Table 110-1 . Although ascites may merely reflect generalized third-space fluid loss in conditions such as congestive heart failure, chronic renal disease, and massive fluid overload, it is more commonly related to intra-abdominal factors that produce peritoneal fluid more rapidly than it can be absorbed. Cirrhosis and neoplasm are the two most common causes of ascites in the Western world. Tuberculosis and cirrhosis are the predominant causes worldwide.
Portal Hypertension Related
Cirrhosis
Alcoholic hepatitis
Fulminant hepatic failure
Heart failure
Constrictive pericardial disease
Budd-Chiari syndrome
Hepatic veno-occlusive disease
Liver metastases
Peritoneal Diseases
Carcinomatosis from ovarian, colon, gastric, pancreatic, hepatic, and other neoplasms
Mesothelioma
Tuberculosis
Fungal infections
Pyogenic infections
Peritonitis
Sarcoidosis
Vasculitis
Eosinophilic gastroenteritis
Whipple’s disease
Miscellaneous Causes
Myxedema
Pancreatic ascites
Chylous ascites
Hemoperitoneum due to trauma or tumor hemorrhage
Ruptured ovarian cyst
Nephrotic syndrome
Malnutrition
Protein-losing enteropathy
Hypoalbuminemia
Meigs’ syndrome
Ovarian hyperstimulation syndrome
| Imaging Modality | Features Suggesting Benign Transudate | Features Suggesting Complicated Ascites |
|---|---|---|
| Ultrasonography | Anechoic collection Compressible collection Follows contours of gut and solid organs Bowel loops freely float in fluid to center of abdomen Seen first in cul-de-sac and Morison’s pouch | Internal echoes (may represent blood, crystals, infection, tumor) Fluid does not conform to available spaces but displaces gut and solid organs Loculation (may represent tumor, infection, adhesion, inflammation) |
| Computed tomography | Uniform low attenuation (0-20 HU) Bowel loops freely float in fluid to center of abdomen | Loculation (may represent tumor, infection, adhesion, inflammation) High fluid attenuation (>30 HU suggests blood but may have hemoperitoneum with fairly normal attenuation) Delayed contrast enhancement (may indicate tumor or infection) |
| Magnetic resonance imaging | Low signal intensity on T1-weighted image Very high signal intensity on T2-weighted image Bowel loops freely float in fluid to center of abdomen | T1 relaxation shorter with exudates due to protein, tumor, or blood Acute blood (<48 hr) has low signal intensity on T1- and T2-weighted images due to deoxyhemoglobin Intermediate-age blood (2-7 days) has high signal intensity on T1-weighted image and low signal intensity on T2-weighted image due to methemoglobin Delayed contrast enhancement (may indicate tumor or infection) Loculation (may represent tumor, infection, adhesion, inflammation) |
Clinical Findings
A small amount of ascites is often asymptomatic, but as the amount of fluid increases, the patient develops a sense of fullness, discomfort, and abdominal distention. With tense ascites, the patient may experience respiratory distress, nausea, vomiting, anorexia, fever, or pain. Weight gain usually accompanies the fluid accumulation, unless it is associated with alcoholism, neoplasm, or poor nutrition, in which case the patient’s weight may remain stable or drop.
The physical diagnosis of ascites is difficult, unless there is at least 1.5 to 2 L of fluid in the peritoneal cavity. Physical examination reveals abdominal distention, with bulging flanks that are dull to percussion or a fluid wave. As little as 300 to 400 mL of fluid can be demonstrated by placing the patient on hands and knees and producing a dull sound by percussion over the dependent abdomen (i.e., the puddle sign). Umbilical hernias, penile or scrotal edema, and pleural effusion are indirect signs of ascites. Flank dullness is the most sensitive sign, and a fluid wave is the most specific sign of ascites on physical examination.
Diagnostic Paracentesis
Diagnostic paracentesis is indicated for any patient who develops ascites for the first time and for patients with chronic ascites who develop fever, encephalopathy, or abdominal pain. Paracentesis for small volumes should be performed under sonographic guidance with an 18- or 20-gauge, plastic-sheathed catheter to avoid injury to the liver, spleen, or gut. With massive ascites, a blind tap can be performed 2 to 3 cm beneath the umbilicus in the midline to reduce the chance of bleeding along the linea alba or at a left-sided McBurney point to avoid injuring an enlarged liver or spleen. Fluid should be analyzed for protein, lactate dehydrogenase, amylase, blood cell count with differential, bacteriologic and cytologic tests, pH, and triglycerides.
Types of Peritoneal Fluid Collections
Transudates
Transudates are clear and colorless fluid collections with a protein content less than 2.5 g/dL and specific gravity less than 1.016. They are most commonly seen in patients with cirrhosis, long-standing heart failure, constrictive pericarditis, chronic renal failure, hypoproteinemia, anasarca, and Budd-Chiari syndrome.
Exudates
Exudates are fluid collections with a density greater than 1.016 and a protein content greater than 2.5 g/dL. They are often yellowish but may be hemorrhagic in patients with metastatic peritoneal infiltration, infection, tuberculosis, or pancreatitis. An ascitic fluid–to–serum lactate dehydrogenase ratio greater than 0.6 suggests malignant disease. A polymorphonuclear leukocyte count greater than 500/mm 3 suggests infection or pancreatic ascites, and a mononuclear cell count larger than 500/mm 3 is commonly seen with tuberculosis. A pH less than 7.35 also indicates tumor, infection, or pancreatitis. A fluid amylase value greater than 1000 U/L and a protein value greater than 3 g/dL are usually associated with pancreatic ascites.
Hemorrhagic Ascites
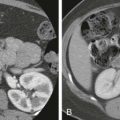
Hemorrhagic ascites usually occurs in the setting of hepatic or splenic trauma resulting from an accident ( Fig. 110-1A ), surgery, or biopsy. Sanguineous ascites is usually caused by malignant disease but may occur in patients with tuberculosis or chronic pancreatitis.

Pus
Pus is often present in the peritoneal cavity in patients with acute peritonitis. It is most often seen in young patients with pneumococcal or hemolytic streptococcal peritonitis. When peritonitis is caused by a perforated viscus ( Fig. 110-1B ), appendicitis, diverticulitis, or tubo-ovarian abscess, signs and symptoms are typically impressive, but the amount of intraperitoneal fluid is relatively small. The presence of more than 500 leukocytes/mm 3 is indicative of infected ascites.
Chylous Ascites
Chylous ascites is a collection of yellowish white, milky fluid that results from obstruction or disruption of lymph flow through the cisterna chyli and thoracic duct. The milky appearance results from the presence of fat (mainly triglycerides in amounts greater than 400 mg/dL) and small amounts of cholesterol and phospholipid. Triglyceride levels may treble after a fatty meal. The causes of chylous ascites include blunt, penetrating, and surgical trauma; malignant infiltration of the cisterna chyli by lymphoma or carcinoma of the pancreas, stomach, colon, or ovary; left subclavian vein thrombosis; and tuberculous lymphadenitis. Cirrhosis, nephrotic syndrome, protein-losing enteropathy, congenital lymphatic disorders, chronic lymphocytic leukemia, sarcoidosis, and extremely high right-sided heart pressure that alters lymph flow are less common causes of chylous ascites. The discovery of milky ascites in an adult mandates a careful search for malignant disease, particularly lymphoma.
Neonatal Ascites
Neonatal ascites may result from posterior urethral valves, perforated bladder, or urethral atresia that produces urine ascites. Ileal atresia, ileal perforation, ileal volvulus, ischemia, cardiac failure, and infections can also result in neonatal ascites.
Pseudomyxoma Peritonei
Pseudomyxoma peritonei is characterized by the massive accumulation of gelatinous, mucinous material in the peritoneal cavity ( Fig. 110-1C ), mesentery, and omentum. It is produced by metastatic parietal and visceral peritoneal implants that are seeded after rupture of benign or malignant mucin-secreting tumors of the appendix or ovary. Less commonly, mucinous tumors of the pancreas, stomach, colon, uterus, bile duct, urachus, omphalomesenteric duct, or pancreas cause pseudomyxoma peritonei.
Bile Ascites
Bile ascites can develop after trauma, cholecystectomy, biliary tract surgery, hepatic surgery, liver biopsy, and percutaneous biliary drainage. These collections typically occur in the right or the left supramesocolic spaces.
Pancreatic Ascites
Virtually all patients with clinically significant pancreatitis have peripancreatic fluid collections, most of which resolve within 6 weeks (see Chapter 96 ). These collections most often occur in the lesser sac and anterior pararenal space. Fluid accumulation in the greater peritoneal sac usually occurs in the setting of severe pancreatitis “burning” the peritoneum, in trauma, or after surgical resection. The cause of pancreatic ascites in all cases is disruption of the pancreatic duct. Endoscopic retrograde pancreatography is diagnostic in these cases. Pancreatic ascites should be part of the differential diagnosis of every patient with chronic ascites who has a history of pancreatitis, alcoholism, or abdominal trauma.
Urine Ascites
Urine ascites usually follows a bladder tear or injury to another portion of the collecting system after direct trauma, seat belt–caused injury, or use of instrumentation. Most urinomas accumulate in the retroperitoneum rather than in the peritoneal cavity.
Cerebrospinal Fluid Ascites
Cerebrospinal fluid ascites and pseudocyst formation account for 4.5% of all ventriculoperitoneal shunt complications. This type of ascites occurs when the peritoneal cavity fails to absorb the fluid or when lymphatic disruption diminishes return of fluid to the bloodstream. If the collection elicits an infectious or inflammatory response, adhesions may develop and cause encystation of the cerebrospinal fluid.
Pathways of Fluid Distribution
A number of factors determine the distribution of fluid in the peritoneal cavity: volume; peritoneal pressures; position of the patient; region of origin; rate of fluid accumulation; presence of adhesions; density of the fluid; and peritoneal, mesenteric, and omental ligaments and reflections. The pathways of intraperitoneal fluid distribution are summarized in Figure 110-2 . Briefly, fluid in the inframesocolic space seeks the pelvis: on the right, through the leaves of the small bowel mesentery, and on the left, through the medial side of the rectosigmoid. After fluid fills the pouch of Douglas, which is the most dependent portion of the peritoneal cavity, it fills the lateral paravesical recesses and then ascends the paracolic gutters. On the left, its cephalad extent is limited by the phrenicocolic ligament. Fluid in the right paracolic gutter, which structure represents the main communication between the upper and lower abdominal compartments, reaches Morison’s pouch and subsequently the right subphrenic space. This is discussed more fully in Chapter 108 .

Radiologic Findings
Chest Radiography
Elevation of the diaphragm, with or without sympathetic pleural effusions (i.e., hepatic hydrothorax), is seen in patients with massive ascites. The cause of the ascites can sometimes be suggested by the chest radiograph. With chylous effusions, a superior mediastinal mass with tracheal deviation may be seen; pericardial calcification or effusion and mediastinal adenopathy are also helpful signs.
In patients with hiatal hernia, massive ascites can cause a mediastinal pseudotumor as fluid passes through the esophageal hiatus into the posterior mediastinum. This pseudotumor is caused by the fact that the stomach remains covered by peritoneum to the level of the gastroesophageal junction, regardless of where this point lies. Sometimes, the ascites may enlarge the hernial sac out of proportion to the size of the gastric pouch and cause dysphagia and produce a retrocardiac mass visible on barium studies. Cyclic negative intrathoracic pressure created during respiration may provide an alternative route for clearance of peritoneal fluid through defects in the tendinous diaphragm.
Plain Abdominal Radiography
Plain radiographs are insensitive for the diagnosis of ascites and have been replaced by cross-sectional imaging. More than 500 mL of fluid is usually required for ascites to be diagnosed on plain radiographs.
Indirect signs of ascites on plain radiographs are often nonspecific or helpful only in the presence of massive peritoneal fluid: diffuse abdominal haziness, bulging of the flanks, indistinct psoas margins, poor definition of the intra-abdominal organs, erect-position density increase, separation of small bowel loops, and centralization of floating gas containing small bowel. The direct signs of ascites are more reliable and specific. In 80% of patients with ascites, the lateral liver edge is medially displaced from the adjacent thoracoabdominal wall (i.e., the Hellmer sign). Obliteration of the hepatic angle (visible in 80% of normal patients) is a close corollary to the Hellmer sign and is related to the fact that fluid in the superior mesocolic space accumulates around the margins of the liver (e.g., Morison’s pouch). In the pelvis, fluid accumulates in the rectovesical pouch, then spills into the paravesical fossae. This fluid produces symmetric densities on both sides of the bladder, producing a dog’s ear or a Mickey Mouse appearance, which is another good sign of ascites. Another direct sign of ascites is medial displacement of the cecum and ascending colon and lateral displacement of the properitoneal fat line. This sign is present in more than 90% of patients with significant ascites.
Barium Studies
When the peritoneal fluid is malignant, barium studies may reveal metastatic serosal implants, mechanical small bowel obstruction, or the fixed colon sign, in which no change in colon position or course is observed on pre-evacuation and postevacuation radiographs. Large fluid collections in the lesser sac may simulate a large retrogastric mass on an upper gastrointestinal examination.
Ultrasonography
Real-time sonography is the easiest and most sensitive technique for detection of ascitic fluid. Volumes as small as 5 to 10 mL can be routinely visualized. Small amounts of fluid are commonly seen in the cul-de-sac of normal women during all phases of the menstrual cycle.
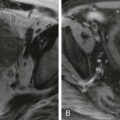
On sonographic examination, uncomplicated ascites appears as a homogeneous, freely mobile, anechoic collection in the peritoneal cavity that demonstrates deep acoustic enhancement ( Fig. 110-3A, B ). Free ascites does not displace organs but typically insinuates itself between them, contouring to organ margins and demonstrating acute angles where the fluid borders the organ. The fluid compresses with increased transducer pressure and shifts with a change in the patient’s position.

Benign ascites can occasionally cause perplexing sonographic artifacts. Perihepatic ascites may cause apparent discontinuity in the diaphragm as a result of reflection of the sound beam at the liver-fluid interface. Echogenic hepatic pseudotumors can be seen in patients with a nodular liver surface. In this artifact, a focal concavity of the liver surface can act as an acoustic lens and transmit sound in a fashion analogous to the way in which a posterior wall of a cyst does. Other sources of confusion are the appendices epiploica of the colon, which can be mistaken for mesenteric or peritoneal metastatic implants.
The smallest amounts of fluid tend to collect in Morison’s pouch and around the liver as a sonolucent band. Fluid may collect anteriorly, as a result of the capillary effect of the narrow space between bowel loops and viscera. In a study of patients with ascites from liver disease, 92% had ascites around the liver, 77% in the pelvis, 69% in the paracolic gutters, and 63% in Morison’s pouch. Although the pouch of Douglas is the most common location for the fluid accumulation of ascites, an overdistended bladder may obscure and displace the fluid to the peritoneal reflection adjacent to the uterine fundus—the so-called triangular fluid cap. In doubtful cases, changes in position produce significant alterations in the location, shape, and size of the fluid. Small amounts of intraperitoneal fluid are readily identified in the cul-de-sac, pouch of Douglas, and Morison’s pouch. With more fluid, ascites can be identified in the paracolic gutters.
With massive ascites, the small bowel loops have a characteristic polycyclic, “lollipop,” or arcuate appearance as they are arrayed on either side of the vertically floating mesentery. The intraluminal gas content and amount of mesenteric fat determine whether the small bowel loops float. In emaciated patients, the entire small bowel mesentery and gut may sink because of the paucity of mesenteric fat. The transverse colon and sigmoid colon usually float on top of the fluid because of the nondependent position of their gas content when the patient is supine. The ascending colon and descending colon do not float because they are fixed in the retroperitoneum. In some patients with massive ascites, the right kidney may also be displaced anteriorly and laterally because fluid in the right paracolic gutter exploits the lack of peritoneal attachment between the right renal fascia and liver or diaphragm.
Certain sonographic patterns suggest that the ascites may be an infected, inflammatory, or malignant exudate ( Fig. 110-3C, D ): coarse internal echoes (i.e., blood), fine internal echoes (i.e., chyle), multiple septa (i.e., tuberculous peritonitis or pseudomyxoma peritonei), loculation or atypical fluid distribution, matting or clumping of bowel loops, and thickening of interfaces between the fluid and adjacent structures. Absence of these findings does not entirely exclude an exudate because these complicated fluid collections may fulfill the sonographic criteria for transudates in one fourth of cases.
In malignant ascites, the bowel loops do not float freely but may be tethered along the posterior abdominal wall or plastered to the liver or other organs, or they may be surrounded by loculated fluid collections. Bowel loops are considered matted or infiltrated if they cannot be moved apart with compression by the transducer or if they fail to separate with fluid despite changes in the patient’s position.
Loculated ascites usually indicates a malignant process or one associated with adhesions and bowel strictures. Loculated ascites usually has rounded or bulging contours, is not compressible with increased probe pressure, does not conform to the margins of organs, and shows no movement with changes in the patient’s position.
Another sign of malignant ascites is the presence of concordant fluid collections in the greater and lesser peritoneal sacs. The significance of this finding is discussed in the section on computed tomography (CT). On sonographic evaluation, these fluid collections produce a butterfly- or wing-shaped lucency separated by the lesser omentum or gastrocolic ligament.
The presence or absence of gallbladder wall thickening is another predictor of benign or malignant ascites (see Fig. 110-3B ). Most patients (95%) with carcinomatous peritonitis have a gallbladder wall less than 3 mm thick. Mural thickening of the gallbladder (>3 mm) is associated with benign ascites in 82% of cases. This thickening of the gallbladder is primarily a reflection of cirrhosis and portal hypertension. Benign, transudative ascites frequently accompanies these disorders.
The sonographic appearances of pseudomyxoma peritonei vary: an exudate with numerous echoes; highly echogenic masses containing numerous scattered cystic spaces; large intraperitoneal, septate, cystic-appearing masses; numerous thick-walled, multiseptate, fluid-filled masses; scalloping of the liver edge; and multiple rounded, echodense masses, which may cast a shadow resulting from calcification.
The sonographic appearance of bilomas is nonspecific, and needle aspiration is essential to confirm the diagnosis. Bilomas are usually anechoic collections that are located adjacent to hepatic or biliary structures, demonstrate acoustic enhancement, and have sharp margins.
The appearance of cerebrospinal fluid ascites is also nonspecific. A small amount of free intraperitoneal fluid is a normal finding in patients with a ventriculoperitoneal shunt and suggests normal shunt function. Its absence does not indicate malfunction. However, a localized fluid collection in association with the tip of the shunt tube is pathologic and implies malfunction.
The sonographic appearance of hemorrhage is highly variable and depends on the frequency of the transducer. Fresh hemorrhage imaged with 2.25- and 3.0-MHz transducers is typically anechoic with increased sound transmission. At 5.0, 7.5, and 10.0 MHz, the clot is intensely echogenic. This echogenicity is transient and usually disappears within 96 hours, as the clot undergoes hemolysis. As the clot organizes, internal echoes are generated that disperse evenly throughout the fluid or layer dependently. Chronic hematomas often have coarse clumps of highly echogenic material. With time, the clots may become completely anechoic seromas.
Although the sonographic findings of hematomas are nonspecific, in the appropriate clinical setting of trauma, acute anemia, blood loss, or pelvic pain, certain findings can be most instructive. For example, the presence of highly echogenic material in the cul-de-sac may be helpful in diagnosis of pelvic hemoperitoneum because most other pelvic fluid collections are predominantly anechoic, with low-level echoes.
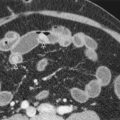
Computed Tomography
Ascites is well demonstrated by CT. Small amounts of ascitic fluid localize in the right perihepatic space, the posterior subhepatic space (i.e., Morison’s pouch), and the pouch of Douglas. When larger amounts of ascites are present, the fluid accumulates in the paracolic gutters, causing progressive centralization of bowel loops. The fluid may accumulate in a triangular configuration within the leaves of the small bowel mesentery or adjacent to bowel loops. A massive amount of fluid distends the peritoneal spaces.
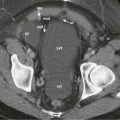
It is usually difficult to characterize the nature of a peritoneal fluid collection on the basis of CT density, but several general rules are helpful. Simple ascites appears as a low-density collection of fluid ( Fig. 110-4A ) with an attenuation value ranging from 0 to 30 Hounsfield units (HU). The density of the ascitic fluid increases with increasing protein content and with exudates. Similarly, acute intraperitoneal hemorrhage can often be distinguished from other fluid collections because it has an attenuation value greater than 30 HU ( Fig. 110-4B ). The CT characteristics of blood within the peritoneal cavity change in a matter of hours or days, in contrast with intracerebral bleeds, in which lysis of blood and consequently CT density evolve during days or weeks. These temporally dramatic changes are related to the impressive fibrinolytic activity of the peritoneum. High-density blood implies that the bleeding is recent or rapid and mandates careful observation of the patient. Several caveats are warranted in interpreting high-attenuation fluid. Delayed enhancement of ascites may follow infusion of a large dose of contrast medium for delayed hepatic CT scanning.