- •
Nuclear imaging with PET and SPECT can be used to study the biologic mechanisms that underlie high-risk atherosclerotic plaque features.
- •
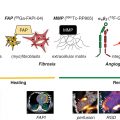
High-risk plaque features targeted by nuclear probes in atherosclerosis imaging research include vascular inflammation, active calcification, apoptosis, hypoxia, neoangiogenesis, and thrombosis.
- •
Although 18 F-FDG PET is the most widely studied and best validated nuclear tracer for imaging vascular inflammation, as a marker of glucose metabolism it lacks inflammatory cell specificity. 18 F-FDG PET is also unreliable for coronary artery imaging because of high background physiologic myocardial uptake.
- •
Several PET tracers have been identified as potential alternatives to 18 F-FDG for imaging vascular inflammation, including 68 Ga-pentixafor and 68 Ga-DOTATATE, which are more specifically targeted to inflammatory cells and have inherently lower myocardial signals.
- •
18 F-NaF binds to developing microcalcifications undetectable by CT in high-risk carotid and coronary atherosclerotic plaques as well as areas of exposed hydroxyapatite in more advanced lesions with established macrocalcification. The ability of this tracer to predict patients with high-risk plaques who are most at risk for recurrent MI is the subject of an ongoing clinical trial.
Introduction
Molecular imaging can uncover key insights about the pathobiology and natural history of atherosclerosis and help identify high-risk features in otherwise stable patients with cardiovascular disease (CVD). Unlike currently employed structural imaging targets in CVD, inflammation and related pathologic processes occurring at the molecular level can provide useful markers of atherosclerotic disease activity to study disease mechanisms, test drug efficacy, and, ultimately, improve CVD risk prediction. In this chapter, we will discuss the range of nuclear tracers that have been examined for marking the molecular signatures of high-risk atherosclerotic plaques and explore the future potential of these novel indicators of disease activity to better inform the clinical management of high-risk patients. Details of the imaging modalities and radiopharmaceuticals discussed in this chapter are described in Chapters 1 , 2 , and 4 .
Rationale for high-risk plaque imaging
Biology of high-risk atherosclerotic plaques
Atheroma, derived from the Greek word athera, meaning “gruel,” is an accumulation of lipid-laden foam cells and necrotic cellular debris arising within an inflammatory milieu in the vascular tunica intima. The term atherosclerosis refers to “hardening” ( skelerosis) of atheromatous lesions, which occurs gradually over time because of the formation of a fibrous extracellular matrix, with subsequent arterial calcification. Although atherosclerosis typically follows a chronic, indolent course, these pathologic manifestations nonetheless result from highly dynamic and heterogeneous active biologic processes that underpin the unpredictable nature of the disease.
Atherosclerotic plaques form predominately at branch points and bends of large and medium-sized arteries that are most susceptible to altered flow dynamics, leading to endothelial cell activation; upregulation of cell-adhesion molecules (e.g., vascular cell adhesion molecule [VCAM]-1); and the margination and inward migration of monocytes, lymphocytes, and other bone marrow–derived immune and progenitor cells.
In the vessel wall, monocytes differentiate into populations of lesional macrophages, including both atherogenic and atheroprotective subsets, which exert plasticity in vivo, being directed by the local cytokine microenvironment, as well as lipid loading, and the cell’s metabolic state. Vascular permeability also allows low-density lipoprotein (LDL) and other apolipoprotein B–containing lipoproteins to accumulate in the subendothelial space, where they undergo modification/oxidation, becoming cytotoxic and chemotactic, thus perpetuating the local vascular inflammatory response. As the lesion progresses, macrophage proliferation becomes more important for plaque growth than monocyte recruitment. Neutrophil extracellular traps also contribute to endothelial dysfunction and may initiate interleukin (IL)-1β/TH17 and type I interferon (IFN) responses, among other functions, leading to further activation of lesional leukocytes and inflammatory cytokine release.
Foam cells, the hallmark of atherosclerosis, are formed when macrophages bearing scavenger receptor A endocytose–modified LDL cholesterol and, to a lesser extent, by T cells, macrophage-like vascular smooth muscle cells, and dendritic cells. Foam cell necrosis and defective efferocytosis lead to the accumulation of oxidized lipids and cellular debris within the necrotic core, and the formation of inflammasome-activating cholesterol crystals and danger-associated molecular patterns. Cell signaling promotes migration of vascular smooth muscle cells to the intima where they undergo phenotypic switching from a contractile to a synthetic phenotype, enabling these cells to secrete collagen and other essential constituents of the protective fibrous cap. Adventitial stem cells, pericytes, and fibroblasts likely also contribute to populations of vascular smooth muscle-like cells in the neointimal lesion. Intimal calcification occurs as part of a healing response to intense inflammation and cell death, involving the release of extracellular calcifying microvesicles, with divergent pathways leading to microcalcification or sheet-like macrocalcification.
The vulnerability to plaque rupture that underlies most myocardial infarctions (MIs) is determined by numerous factors, including fibrous cap thickness and degree of inflammation. Fibrous cap thinning results from an imbalance between proteolytic enzymes, namely matrix metalloproteinases, and collagen and extracellular matrix synthesis. Inflamed regions of the plaque are most affected by cap thinning, including the plaque shoulder, which tends to be an area affected by low wall shear stress, and vascular smooth muscle cell apoptosis and senescence. Among other factors that contribute to the risk of plaque rupture are structural stresses governed by the material properties of plaque components, including microcalcifications, intraplaque hemorrhage causing rapid plaque expansion and the accumulation of free cholesterol and inflammatory cell infiltration, coronary vasospasm, and sudden emotional or physical stress.
Exposure of the lipid core contents to blood through a ruptured plaque culminates in the formation of intraluminal thrombus through activation of platelets and the clotting cascade. MI arising from atherosclerotic plaque rupture occurs most commonly during the morning hours just after waking because of abrupt changes in systemic hemodynamics, sympathetic tone, and circadian variations in platelet aggregation, hormone release, and fibrinolytic activity. Nevertheless, plaque rupture is also frequently subclinical, with advanced plaques showing evidence of multiple ruptures and repair resulting in progressive luminal narrowing.
Histologic descriptions of high-risk plaque features
Pathologic series of atherosclerotic plaques have reported several structurally distinct lesion types, with varying complexity and with differing propensities to instigate acute clinical events, ranging from the initial intimal lesion to advanced fibroatheromas. Autopsy studies performed more than 30 years ago revealed that sudden cardiac ischemic death is most often the result of plaque rupture. Thrombus associated with plaque erosion and calcific nodules are less frequently identified causes of MI where there is no histologic evidence of plaque rupture. However, plaque erosion may be more prevalent in certain MI patient populations than previously recognized because of the plaque stabilizing effects of statins. The most common plaque type identified as the cause of sudden coronary death is a thin, ruptured fibrous cap with heavy macrophage infiltration and few smooth muscle cells, a large necrotic core, and an intraluminal thrombus. Because nonthrombosed thin-cap fibroatheromas bear the closest histologic resemblance to high-risk ruptured plaques, they have been identified as the most likely “vulnerable” precursor lesion. These high-risk plaques are also associated with speckled calcification patterns, fragile new vessel formation, and positive arterial remodeling; this allows a high burden of disease in the vessel wall to remain undetectable by luminal angiography.
Imaging to identify high-risk plaques
Although CVD accounts for more deaths than any other cause worldwide, most individuals with CVD will never experience overt clinical symptoms. Moreover, most MIs occur in individuals without prior knowledge of their underlying disease. In clinical practice, coronary disease often remains undiagnosed until patients are at an advanced stage in the disease and present with symptoms of stable angina or experience an MI. Risk factor modification and therapeutic interventions that are needed to improve long-term prognosis are often only initiated at this late stage.
Clinical risk assessment algorithms and diagnostic pathways have reasonable sensitivity and specificity to diagnose CVD, yet risk prediction remains a significant challenge. In fact, although detection of angiographic stenosis severity and myocardial ischemia confer adverse prognosis, landmark clinical trials examining ischemia-guided percutaneous coronary intervention for stable angina in patients without significant left main coronary artery disease have yielded mixed results, with some studies showing improved outcomes and others failing to show significant reductions in MI or death.
Similarly, although it is well known that certain plaque types are more likely to rupture than others, identification of individual plaques with high-risk morphologic features by intravascular imaging or coronary computed tomography (CT) angiography (CCTA) in large, prospective clinical trials have demonstrated disappointingly low positive predictive value for future coronary events. A post hoc analysis of 5-year clinical outcome data from SCOT-HEART (Scottish COmputed Tomography of the HEART) showed that although individuals with adverse plaque features identified by CCTA were three times more likely to develop coronary death or nonfatal MI, this association was not independent of plaque burden assessed by the coronary artery calcium score. Although plaque burden appears to be the strongest indicator of future coronary events, it neither predicts the timing nor location of acute plaque events.
Role of novel multimodal imaging assessments
Because mechanisms underlying cardiovascular events are complex and unpredictable by any current imaging marker of disease severity, there remains a need to consider the wider constellation of plaque, patient, and environmental level factors contributing to cardiovascular events. Adoption of more comprehensive multimodal imaging approaches, which consider not only the conventional markers of disease severity but also novel assessments of the biomechanical environment, computer-identified radiomic plaque and perivascular adipose tissue features, and molecular imaging–derived measures of local and systemic disease activity, can enable better understanding of disease mechanisms and strategies for CVD risk stratification.
Molecular probes for imaging atherosclerotic disease activity
Molecular imaging has been applied in atherosclerosis research to quantify several important markers of disease activity in patients with high-risk plaques. Among the biologic processes targeted by molecular imaging approaches in atherosclerosis are vascular inflammation, active calcification, apoptosis, hypoxia, neoangiogenesis, and thrombosis ( Fig. 12.1 ). Aside from the radionuclide imaging methods discussed in this chapter, a range of other molecular imaging platforms have been tested for plaque imaging, including magnetic resonance imaging (MRI) with ultrasmall superparamagnetic iron oxide or gadolinium-labeled tropoelastin-specific contrast agents, CT-based nanoparticles, contrast-enhanced ultrasound with antibody-conjugated microbubbles, and catheter-based near-infrared fluorescence imaging. Of all the experimental molecular imaging methods used in atherosclerosis, 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has been the most widely studied.

18 F-FDG PET imaging of vascular inflammation
Vascular inflammation, driven by innate and adaptive immune responses to arterial injury and lipid dysregulation, serves as a common end point linking most CVD risk factors to atherosclerotic disease progression and its clinical sequelae. Although 18 F-FDG PET has been the most commonly applied imaging marker of vascular inflammation, focus is beginning to shift toward other more specifically targeted PET ligands because of the limitations of plaque imaging using 18 F-FDG.
18 F-FDG signals in atherosclerosis: A critical appraisal
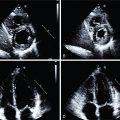
As discussed in Chapter 4 , 18 F-FDG is a radiolabeled glucose analog used commonly in PET imaging for a variety of diagnostic purposes. After initial preclinical and retrospective descriptions of vascular 18 F-FDG signals, a prospective study of eight patients with symptomatic carotid atherosclerosis identified increased 18 F-FDG signals originating from culprit arteries, with autoradiographic tracer accumulation in macrophage-rich plaque regions. Strong correlations between in vivo carotid artery 18 F-FDG signals and macrophage density in excised carotid plaques has been subsequently confirmed by numerous studies ( Fig. 12.2 ).

Moreover, in vitro studies have shown that 18 F-FDG signals in macrophages are most avid during early foam cell formation, with greater glycolytic activity and tracer uptake occurring in response to oxidized LDL and other proinflammatory macrophage stimuli. Indeed, a study examining tracer uptake in polarized THP-1–derived macrophages in vitro demonstrated the accumulation of 18 F-FDG in proinflammatory M1-like macrophages to be sevenfold higher than alternatively activated M2-like macrophages. In another study, the degree of 18 F-FDG uptake in human monocyte–derived macrophages was found to be comparable to human glioblastoma and pancreatic carcinoma cells, which are known to be detectable by PET imaging.
In hyperlipidemic rabbits, aortic 18 F-FDG signals have been shown to localize and quantify the extent of foamy macrophage infiltration. Carotid 18 F-FDG uptake in patients has also been correlated with gene expression markers of glycolysis (GLUT-1 and hexokinase type 2), inflammation (CD68 and IL-18), collagen protease activity (cathepsin K and matrix metallopeptidase-9), and microvessel density (CD34). Moreover, leukocyte trafficking to sites of carotid and aortic plaque inflammation detected by 18 F-FDG PET have also been observed using single photon emission computed tomography (SPECT) with 99m technetium (Tc) labeling of autologous peripheral blood monocytes ( Fig. 12.3 ).

Nevertheless, it is evident that vascular 18 F-FDG signals are not solely representative of macrophage-related inflammation because plaque microvascularization, hypoxia, and nonspecific tracer uptake by other glucose metabolizing vascular and inflammatory cells (e.g., neutrophils, lymphocytes, and fibroblasts) also contribute significantly to signal intensity. In chronic inflammatory lesions, macrophages adapt to the low oxygen concentration by increasing glycolytic metabolism and maintaining a dynamic and linear relationship between energetics and inflammatory activity. One study showed that exposure to hypoxic conditions, but not proinflammatory cytokines, led to increased 3 H-FDG tracer uptake in macrophages and foam cells when examined in vitro. In contrast to these findings in macrophages, both vascular smooth muscle cells and endothelial cells demonstrated significant increases in tracer uptake after treatment with IFN-γ, tumor necrosis factor (TNF)-α, and IL-1β in this study.
These results are supported by data from other studies confirming a moderately strong relationship between hypoxia inducible factor (HIF)-1α expression and 18 F-FDG uptake in carotid plaques and showing increased tracer uptake in vascular smooth muscle cells in transgenic atherosclerotic minipigs. The latter study also found that, in the minipig model, the relationship between 18 F-FDG and macrophage density was dependent on total arterial volume. Despite these observations that raise important questions about the interpretation of vascular 18 F-FDG PET signals in certain situations, there remains robust evidence to support its use as a marker of plaque metabolic activity in molecular imaging research.
18 F-FDG uptake in high-risk plaques
In patients with carotid artery disease, 18 F-FDG uptake has been shown to accurately differentiate culprit and high-risk atherosclerotic plaques from lower-risk arteries. A meta-analysis of data from 539 patients in 14 studies showed that 18 F-FDG uptake was significantly higher in culprit carotid arteries than nonculprit arteries in patients with transient ischemic attack (TIA) or stroke, with a standard mean difference of 0.94. In one study, 18 F-FDG uptake originating from nonstenotic carotid lesions in vascular territories was compatible with presenting symptoms of patients with TIAs, further demonstrating the potential value of imaging plaque activity over stand-alone anatomic imaging. Similarly, another study showed that although 18 F-FDG PET could identify symptomatic from asymptomatic carotid plaques, this distinction was not apparent using ultrasound measures of plaque echolucency.
Carotid inflammation measured by 18 F-FDG is significantly associated with many known high-risk features, including distal microembolic signals on transcranial Doppler, positive remodeling and low attenuation assessed by CT, and the presence of complicated plaques with lipid-rich necrotic cores and intraplaque hemorrhage identified by MRI. A relationship between carotid 18 F-FDG uptake and vascular permeability assessed by dynamic contrast-enhanced (DCE) MRI has also been demonstrated in symptomatic arteries. Moreover, another study showed increased carotid 18 F-FDG uptake in macrophage-rich plaques induced by low wall shear stress because of the placement of a flow-restricting cuff in ApoE -/- mice fed a cholesterol-rich diet.
As a barometer of systemic vascular inflammation, aortic 18 F-FDG signals might also help identify patients at risk for developing high-risk coronary plaques. For example, one study showed that aortic 18 F-FDG uptake was associated with an increased number of lipid-rich coronary plaques identified by optical coherence tomography in patients with MI. Aortic 18 F-FDG uptake was also associated with noncalcified plaque burden and the prevalence of low attenuation high-risk plaques on CT angiography in a cross-sectional study of 215 patients with chronic systemic inflammation due to psoriasis. Indeed, multiple studies have also shown that 18 F-FDG signals marking inflammation do not tend to colocalize with plaque macrocalcification, which typically arises at a later “burnt-out” stage in the disease.
Arterial 18 F-FDG signal reproducibility and disease thresholds
A good imaging biomarker will be easy to detect and provide robust, reproducible measurements, with low intrinsic signal variability under physiologic conditions. Measurements of vascular 18 F-FDG uptake are highly reproducible and exhibit low short-term interscan variability in multiple vascular territories, including the carotids, aorta, iliac, and femoral arteries. Moreover, data from the placebo-control arms of several clinical drug trials have shown that vascular 18 F-FDG signals remain reasonably constant over periods of 3 to 6 months in patients without recent CVD events. , In one study, elevated 18 F-FDG signals were prevalent in roughly one-third of patients with carotid atherosclerosis detected by ultrasound and in 10% of those without. In another study, which compared carotid and aortic 18 F-FDG signals in patients with known CVD, in patients at risk for CVD, and in healthy control subjects, PET signals accurately differentiated the three groups, with excellent intra- and interobserver interscan agreement demonstrated on repeat imaging at 3 weeks. The upper threshold for physiologic 18 F-FDG uptake in healthy control subjects derived in this study using the 90th percentile of the average target-to-background ratio of the whole vessel was 1.84 for the carotid arteries and 2.68 in the aorta.
Mechanistic insights gained from 18 F-FDG imaging
In the context of vascular imaging research, 18 F-FDG can be used to examine disease mechanisms. For example, 18 F-FDG PET has helped to characterize networks of local and systemic inflammation at play in high-risk patients with atherosclerosis, including the cardio-splenic and heart-brain axes, and cross talk between perivascular adipose tissue and arterial inflammation.
Systemic vascular inflammatory networks
Systemic inflammation measured in the carotid arteries and aortas of patients after MI using 18 F-FDG has been linked to metabolic activity in the bone marrow and spleen as well as C-reactive protein, proinflammatory gene expression in circulating leukocytes (CD16 and TLR-4), and downstream postinfarct myocardial inflammation. In stable CVD patients, higher splenic and bone marrow 18 F-FDG uptake has also been demonstrated compared with healthy control patients, indicating chronic activation of the hematopoietic system driving low-grade inflammation in atherosclerosis. These clinical observations confirm findings of preclinical studies performed in mouse models of atherosclerosis. Moreover, increased metabolic activity in the spleen has also been identified as an independent marker of subsequent CVD events in a group of 464 patients who had previously undergone 18 F- FDG PET imaging.
Further insight is provided by another report of two parallel studies, which concluded that abnormal amygdalar activity in patients with chronic psychologic stress associated with bone marrow activation and arterial inflammation measured by 18 F-FDG PET, conferred an increased risk for CVD events. Abnormal amygdalar activity and vascular inflammation in individuals subjected to chronic noise exposure has also been linked to the occurrence of major adverse cardiac events ( Fig. 12.4 ).

Adipose tissue and vascular inflammation
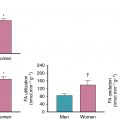
Measures of perivascular fat attenuation and its radiomic profile identified by CCTA are associated with vascular inflammation and adverse CVD outcomes. In the initial description of the CT fat attenuation index, this method was validated against 18 F-FDG uptake in subcutaneous adipose tissue. Greater 18 F-FDG uptake in pericoronary adipose tissue (PCAT) has also been shown in patients with stable coronary artery disease compared with matched healthy control subjects. Significant positive correlations have also been observed between subcutaneous, pericardial, and abdominal visceral adipose tissue and arterial 18 F-FDG uptake , as well as an inverse correlation between 18 F-FDG uptake in supraclavicular brown adipose tissue and the aorta. In these studies, measures of 18 F-FDG uptake in adipose tissue were significantly associated with both increased (visceral fat) and reduced (brown adipose tissue) risk for CVD events. The clinical utility of measuring adipose tissue inflammation as a prognostic imaging biomarker, and the specific contributions of vascular inflammation to this link, remains to be fully elucidated.
18 F-FDG PET as a readout of drug efficacy
Imaging markers can be used as end points in research studies to help distinguish novel therapies that are most likely to succeed in larger-scale clinical outcome trials. Inflammation imaging is particularly relevant for this application because contemporary data from randomized, controlled clinical outcome studies indicate that targeted inhibition of the IL-1β inflammatory pathway with monoclonal antibody therapy, or more broad dampening of inflammatory cell function with colchicine, can significantly improve the prognosis in patients with CVD. In fact, many more immunomodulatory targets are being explored for therapeutic gain in CVD. 18 F-FDG PET has proven to be a useful imaging end point in several clinical CVD drugs trials monitoring early effects of statins and other drugs on vascular inflammation, where antiinflammatory effects (or lack thereof) were predictive of clinical efficacy ( Table 12.1 ).
| Authors | DESIGN a | Population | n | Drug | Duration | Arteries | Findings | Consistent With Outcome Data? | Notes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | B | P | |||||||||
| Tahara N, et al. (2006) | ✓ | Cancer screening, incidental arterial 18 F-FDG uptake | 43 | Simvastatin 5–20 mg once daily | 3 months | Carotid + aorta | ↓ SUV | Yes | No reduction in TBR with dietary modification alone | ||
| Ishii H, et al. (2010) | ✓ | Stable angina, TC ≥220 mg/dL and/or LDL ≥140 mg/dL, not receiving statins | 30 | Atorvastatin 5 or 20 mg once daily | 6 months | Aorta + femoral | ↓ TBR | Yes | No reduction in TBR in the lower-dose group | ||
| Wu Y-W, et al. (2011) | CVD, LDL ≥100 mg/dL, statin naïve | 43 | Atorvastatin 40 mg once daily | 3 months | Aorta + femoral | ↓ TBR | Yes | TBR reduction marginally correlated with changes in MMP-9 levels | |||
| Tawakol A, et al. (2013) | ✓ | ✓ | CVD or RFs, not receiving high-dose statin, arterial TBR ≥1.6 | 67 | Atorvastatin 10 or 80 mg once daily | 3 months | Carotid + aorta | ↓ TBR | Yes | Dose-dependent reduction in TBR from 4 weeks | |
| Lo J, et al. (2015) | ✓ | ✓ | ✓ | HIV infection, subclinical CVD on CT coronary angiogram, LDL <130 mg/dL, arterial TBR ≥1.6 | 40 | Atorvastatin 20 mg to 40 mg once daily | 12 months | Aorta | No change in TBR relative to placebo | N/A | Reduction in CT-derived noncalcified plaque volume and the number of high-risk plaques |
| Singh P, et al (2016) | ✓ | ✓ | CVD or RFs, LDL ≥60 mg/dL and TG <350 mg/dL, statin naïve or low-dose statins, arterial TBR ≥1.6 | 83 | Atorvastatin | 3 months | Left main coronary + carotid + aorta | ↓ TBR | Yes | Reduction in left main coronary 18 F-FDG uptake in patients with high-risk CT features | |
| van der Valk FM, et al. (2016) | Ankylosing spondylitis | 44 | Atorvastatin 40 mg | 3 months | Carotid | ↓ TBR | N/a | 20% higher baseline TBR in patient with ankylosing spondylitis than healthy matched control subjects | |||
| Fayad ZA, et al. (2011) | ✓ | ✓ | ✓ | CVD, diabetes, or Framingham risk score >20%; TG ≤400 nm/dL; LDL ≤100; arterial TBR ≥1.6 | 130 | Dalcetrapib 600 mg once daily | 6 months | Carotid + aorta | No change in TBR relative to placebo | Yes | Reduction in MRI-derived total vessel area after 24 months |
| Tawakol A, et al. (2014) | ✓ | ✓ | ✓ | Stable CVD on statin therapy | 83 | Rilapladib 250 mg once daily | 3 months | Carotid + aorta | No change in TBR relative to placebo | Yes | No change in TBR compared with placebo in post hoc analyses of arteries with plaque on MRI and TBR ≥1.6 |
| Elkhawad M, et al. (2012) | ✓ | ✓ | ✓ | CVD, receiving stable statin therapy | 99 | Losmapimod 7.5 mg once or twice daily | 3 months | Carotid + aorta | No change in TBR relative to placebo in primary end point | Yes | Reduction in average TBR in active segments (TBR ≥1.6) |
| Emami H, et al. (2015) | ✓ | ✓ | ✓ | CVD on low-intensity statins, LDL 70–130 mg/dL, arterial TBR ≥1.6 | 72 | BMS–582949 100 mg once daily or atorvastatin 80 mg once daily | 3 months | Carotid + aorta | No change in TBR relative to placebo for BMS–582949 | Yes | Reduction in TBR in active segments (TBR ≥1.6) with atorvastatin |
| Stiekema LCA, et al. (2019) | ✓ | ✓ | ✓ | LDL >100 mg/dL, Lp (a) >50 mg/dL, arterial TBR >1.6 | 129 | Evolocumab 420 mg once monthly | 16 weeks | Carotid + aorta | No change in TBR relative to placebo | No | Reduction in LDL by 61% and lipoprotein (a) by 14% relative to placebo |
| Hoogeveen RM, et al. (2019) | ✓ | ✓ | ✓ | CVD or familial hypercholesterolemia, LDL >100 mg/dL, not receiving statins | 50 | Alirocumab 150 mg once every 2 weeks | 14 weeks | Carotid | ↓ TBR | Yes | No differences in plasma interleukin levels, monocyte phenotype, or functional monocyte migration |
| Hsue PY, et al. (2018) | Treated HIV infection, CVD, or RFs | 10 | Canakinumab 150 mg single dose | 12 weeks | Aortic | ↓ TBR | Yes | Reductions in inflammatory biomarkers (high-sensitivity CRP, IL-6, and sCD163) and bone marrow 18 F-FDG uptake | |||
| Mizoguchi M, et al. (2011) | ✓ | Impaired glucose tolerance or diabetes with carotid atherosclerosis and 18 F-FDG uptake | 56 | Pioglitazone (15–30 mg) or glimepiride (0.5–4.0 mg) | 4 months | Carotid + aorta | ↓ TBR with pioglitazone | Yes | Increased HDL associated with attenuation of plaque inflammation | ||
| Maki-Petaja KM, et al. (2012) | Rheumatoid arthritis eligible for anti TNF-α therapy | 51 | Etanercept or adalimumab | 8 weeks | Aortic | ↓ TBR | N/a | Baseline aortic TBR greater in patients with rheumatoid arthritis than control subjects with CVD | |||
Dampening of arterial inflammation after treatment with statins has been observed in several studies using 18 F-FDG PET, which have demonstrated this response to be dose-dependent and closely related to changes in serum cholesterol and inflammatory biomarkers. In one study, 18 F-FDG signal reduction in the left main coronary artery following treatment with atorvastatin was more pronounced in arteries with noncalcified or partially calcified plaque than those without. A meta-analysis of seven studies provides further confirmation that statins reduce arterial wall inflammation assessed by 18 F-FDG PET.
Numerous other CVD drugs have been tested using 18 F-FDG PET, including the cholesteryl ester transfer protein inhibitor dalcetrapib; the Lp-PLA 2 inhibitor rilapladib; the p38 mitogen-activated protein kinase inhibitors losmapimod and BMS-582949; , and the proprotein convertase subtilisin-kexin 9 inhibitors evolocumab and alirocumab. Importantly, lack of significant reductions in arterial inflammation measured by 18 F-FDG in these studies of dalcetrapib, rilapladib, and losmapimod predicted the negative results of subsequent clinical outcome trials. Although evolocumab also did not lower arterial inflammation in a randomized, double-blind, placebo-controlled study of 129 patients, the persistence of lipoprotein (a) elevation after drug treatment was thought to be a confounding factor in this study. Indeed, treatment with alirocumab significantly decreased carotid 18 F-FDG signals in another study of patients with increased CVD risk who were not selected for inclusion to the study because of elevated lipoprotein (a) ( Fig. 12.5 ). Additionally, a single-arm pilot study demonstrated a reduction in arterial inflammation among individuals with treated human immunodeficiency virus (HIV) and established CVD or risk factors given a single injection of the IL-1β antagonist, canakinumab, a drug that has been shown to be associated with a reduction in CVD events.