Chemotherapy is associated with cardiovascular injury, including the development of a cardiomyopathy and vascular remodeling. Cardiac magnetic resonance (CMR) is sensitive to detect not only established morphologic and functional abnormalities but also early, potentially reversible, signs of myocardial injury. It robustly detects and quantifies myocardial edema, inflammation, and focal fibrosis, as well as interstitial fibrosis and vascular remodeling. These capabilities support the role of CMR as an excellent tool for evaluating cardiotoxicity. Novel CMR markers may even enhance patient management by facilitating the early detection of reversible myocardial tissue remodeling before classic morphologic and functional changes appear.
Key points
- •
Several strengths of cardiac magnetic resonance (CMR) imaging, including its ability to accurately assess cardiac morphology, function, and scar, as well as myocardial tissue remodeling, have been supporting its use to investigate myocardial injury in cancer survivals.
- •
Because CMR incorporates several different types of imaging strategies, it provides a very comprehensive evaluation of the cardiovascular system, which is very helpful in patients with cancer.
- •
Novel CMR techniques, incorporating T1 and T2 mapping, have the potential not only to improve the current knowledge of cardiotoxicity, but also has the promising capability to detect early markers of left ventricular remodeling, which may facilitate development of prevention and therapeutic interventions.
Introduction
There have been marked improvements in outcomes among patients diagnosed with cancer. These improvements are related, in part, to the improved understanding of cancer biology leading to the development of more effective therapies. However, as the number of cancer survivors increases, there has been a renewed focus on the potential cardiotoxic effects of cancer therapies. In addition, the types of therapies available for cancer treatment have expanded from traditional radiation and chemotherapy to targeted and immune therapies. There are more than 15 million cancer survivors in the United States, and this number is projected to increase. Both preexisting risk factors for cardiovascular (CV) disease and possible cardiotoxic effects of cancer therapy contribute to enlarge the likelihood of CV disease development and exacerbation to these individuals. Moreover, the number of older patients with multiple risk factors for CV disease who receive a diagnosis of a malignancy has considerably increased during the past 3 decades. The combination of the increased complexities of these cancer therapies, the increased number of cancer survivors, and the recognition of the increased risk of CV disease among cancer survivors has led to the development of a specialty called cardio-oncology or onco-cardiology.
CV complications may occur after many types of cancer therapies, including traditional chemotherapy, radiotherapy, and targeted and immune therapy regimens. The manifestations of CV disease among patients with cancer are broad and although cardiac injury is more frequently observed in the myocardium as left ventricular (LV) systolic dysfunction, it also may appear at other heart structures such as valves, coronary arteries, pericardium, great arteries, and electrical system.
Despite advances, the effective detection and quantification of cancer therapy–related cardiotoxicity is challenged by several factors. First, in many cases, cardiac dysfunction occurs only after many years of chemotherapy and in the absence of clinical symptoms, confounding its association with cancer therapy. In addition, although the definition of cardiotoxicity has proven its clinical utility (a decrease in LV ejection fraction [LVEF] of ≥5% to <55% in the presence of symptoms of heart failure (HF) or an asymptomatic decrease in LVEF by ≥10% to <55%), this definition relies exclusively on longitudinal monitoring of LV systolic function and ejection fraction, commonly performed by standard transthoracic echocardiogram, which has several limitations, such as poor reproducibility, lack to provide tissue characterization and to identify LV remodeling beyond systolic dysfunction. Finally, reductions in LVEF frequently take place within the normal range, indicating that subtle myocardial injury may occur before LV dysfunction.
Consistent data have established that cardiac magnetic resonance (CMR) is one of the most accurate imaging modalities for the assessment of cardiac toxicity and adverse LV remodeling. Its ability to assess cardiac morphology and function is highly accurate and reproducible. CMR also integrates different types of imaging sequences (eg, cine imaging for morphology, T2-weighted imaging for myocardial edema, perfusion for ischemia assessment, late gadolinium enhancement [LGE] for scar, and tagging for myocardial strain imaging), providing a very comprehensive evaluation of the LV remodeling associated with cancer therapy. More recently, different groups have applied more novel sequences to characterize the effects of cancer therapy, such as the use of T1 and T2 mapping techniques; providing not only data regarding the early abnormalities in myocardial tissue composition, but also improving the current mechanistic understanding of cancer-related cardiotoxicity.
Mechanisms of the most common chemotherapy-induced cardiotoxicity
Anthracyclines
Anthracyclines are commonly implicated as a cause of CV toxicity among patients with cancer. The risk of HF with anthracyclines is related to cumulative dose, with the risk increasing with higher cumulative doses. The cardiotoxicity of anthracyclines is far higher than the rates of clinical HF. Specifically, rates of subclinical cardiotoxicity are higher, occurring even with lower cumulative doses, particularly when more than 350 mg/m 2 . In a retrospective analysis, Swain and colleagues identified an increased risk of cardiotoxicity even with doses previously considered safe (≤300 mg/m 2 ). Once HF is established, Mortality from HF secondary to anthracycline therapy ranges from 30% to 70%. There may be a long latency period between anthracycline and clinical HF, especially among young patients; however, among adults, toxicity generally appears early, with 90% of cases occurring within the first year. The cardiotoxicity of anthracyclines is likely a multifactorial process, related to oxidative stress, mitochondriopathy, changes in iron and calcium homeostasis, and in respiratory chain components. Zhang and colleagues proposed a unifying mechanism of cardiotoxicity, implicating topoisomerase-IIβ as an essential driver in this mechanism, because in its presence, doxorubicin activates the DNA response and apoptosis pathways and affects oxidative phosphorylation and mitochondrial biogenesis in cardiomyocytes.
Trastuzumab
Trastuzumab is a monoclonal antibody and inhibits the human epidermal growth factor by the receptor tyrosine-protein kinase erbB-2 pathway. ErbB-2 is also present in cardiomyocytes and downstream pathways that regulate apoptosis, mitosis, cell hypertrophy and elongation, cellular adhesion, angiogenesis, and sensitivity to adrenergic signaling, being potentially deleterious to myocardial tissue. Trastuzumab has been associated with an increased incidence of cardiac dysfunction of 3% to 7%. The risk rises with concomitant use of paclitaxel (13%) and even more when concomitantly administered with anthracyclines and/or cyclophosphamide (27%). Trastuzumab-induced cardiotoxicity is not dose-dependent (as when determined by anthracyclines) but is often reversible, although it is difficult to predict which patients are at risk of developing toxicity.
Mitoxantrone
Mitoxantrone, an anthracenedione agent, is a DNA-topoisomerase II inhibitor with antineoplastic activity and potent anti-inflammatory and immunomodulating properties. It acts intercalating into DNA, reducing DNA repair and interfering with RNA synthesis. Mitoxantrone is associated with cumulative dose-related cardiotoxicity, and myocytes exposed to it experience similar alterations at electron microscopy to those seen with anthracycline. Mitoxantrone-induced cardiotoxicity manifests as systolic and diastolic dysfunction. It is recognized that approximately 2% of patients with cancer treated with drug will develop cardiotoxicity. Although this condition is asymptomatic in most cases, it may transition to congestive HF–related symptoms and may transition to congestive cardiac failure, increasing the risk of death.
Cyclophosphamide
Cyclophosphamide is a nitrogen mustard-alkylating agent with potent antineoplastic, immunosuppressive, and immunomodulatory properties. Although the precise mechanism of cyclophosphamide-induced cardiac toxicity has not been entirely established, the metabolites can cause oxidative stress and endothelial damage. Cyclophosphamide cardiac toxicity is associated with cumulative doses and typically manifests as a fulminant myocarditis.
Tyrosine Kinase Inhibitors
Sunitinib targets the vascular endothelial growth factor molecular pathway through tyrosine kinase inhibition. A recent review indicated that sunitinib was the preferred initial therapeutic option for metastatic renal cell carcinoma. However, sunitinib affects the AMP-activated protein kinase and platelet-derived growth factor receptor, which are critical for cardiomyocyte function and survival, leading to hypertension, LV dysfunction, and HF.
Initial management
Cardiac complication after cancer therapy may manifest in different ways, including cardiac systolic dysfunction, cardiac ischemia, arrhythmias, pericarditis, and electrical repolarization abnormalities. A close interaction and cooperation between oncology and cardiology is required to improve care of many patients with cancer at risk of developing cancer-related cardiac complications. Patients considered for antineoplastic therapy should undergo initial CV evaluation to diagnose preexisting cardiac diseases, including physical examination, electrocardiogram, and analysis of ventricular function, identifying those with conditions that can be readily treated or might require more surveillance. Serial imaging assessment of cardiac function is suggested, even in asymptomatic patients, as discontinuation of cardiotoxic chemotherapy might allow reversible improvement in cardiac function when LV systolic dysfunction is identified in this group.
Cardiac magnetic resonance imaging
Morphologic and Functional Parameters
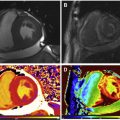
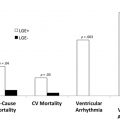
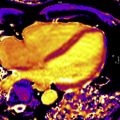
CMR is the method of choice for assessing changes in cardiac morphology and function caused by cancer treatment and cardiotoxicity. Its high contrast-to-noise ratio, superior accuracy, and excellent reproducibility allow detection of slight changes that may benefit from preventive therapies. Both LV and right ventricle (RV) volumes and mass can be precisely obtained using standard cine CMR images, independently of any assumption of ventricle shape and the degree of remodeling. Commonly used steady-state free-precession (SSFP) pulse sequences, which deliver high signal-to-noise and tissue-to-blood contrast ( Fig. 1 ), provide precise data on both global and regional wall motion, capturing even subtle functional changes. Drafts and coworkers 15 demonstrated that CMR cine images were able to detect early and significant abnormalities in cardiac structure and function secondary to cardiotoxicity therapy, even after moderate to low doses of anthracycline chemotherapy. Performing a series of CMR examinations, before and up to 6 months after anthracycline therapy, the investigators showed that reduction in LVEF occurred as early as 1 month after chemotherapy initiation, identifying individuals at high risk to maintain significant later decline in LV function. Interestingly, they also demonstrated that the decrease in LV function was associated with LV enlargement, highlighting the potential negative effect of anthracycline-based chemotherapy on systolic function. Using LGE imaging, new areas with infarcts or scar were not seen, suggesting that anthracycline-induced cardiotoxicity appeared not to be caused by myocardial ischemia or myocardial infarction. A study comparing different imaging approaches for screening adult survivors of childhood cancer treated with anthracycline chemotherapy and/or radiation therapy, has shown that 2-dimensional (2D) echocardiogram, using the biplane method, may not achieve a clinically reasonable accuracy for detecting LVEF less than 50% measured by CMR, demonstrating in this scenario limited sensitivity (25%) with high rates of false-positives (75%). Although 3D echocardiogram may improve sensitivity (53%), this approach also fails to accomplish results comparable to CMR imaging. LV mass can also change during cancer therapy and especially after anthracycline-based chemotherapy. Nearby 50% of childhood cancer survivors have been shown to have LV mass ≥2 standard deviations below the mean normative values. Neilan and colleagues, examining 91 individuals with decreased LVEF after anthracycline-based chemotherapy at a median follow-up 88 months, found that indexed LV mass measured by CMR had a negative correlation with the cumulative given dose of anthracycline ( Fig. 2 A). In the same study, LV mass was shown to be a strong predictor of major CV events, and patients with indexed LV mass less than 57 g/m 2 had significantly higher rates of CV deaths, appropriate implantable cardioverter-defibrillator therapies, and admissions for decompensated HF compared with those with indexed LV mass ≥57 g/m 2 ( Fig. 2 B). A recent study, using novel CMR markers of myocardial tissue remodeling, indicates that LV atrophy could be accounted not only for the expansion of the extracellular space, but also by a reduction in cardiomyocyte size. In addition, Jordan and colleagues suggested not only that the decrease in LV mass occurs early after anthracycline initiation, but also may take place regardless of factors that increase myocardial wall tension and stress. They also showed that a reduction in LV mass was associated with worsening HF symptoms.


Although few observations have specifically focused on the effects of cancer therapy on RV, recent reports have demonstrated significant decline in RV systolic function after anthracycline alone or in combination with trastuzumab. RV failure has been associated with significant morbidity and mortality in patients with HF with both reduced and preserved ejection fraction. Because the RV is a thin and complex structure compared with the LV, noninvasive imaging may be challenging. CMR has been shown to be very accurate and reproducible to assess RV volumes and function, having several advantages over 2D echocardiography, including outstanding spatial resolution, volumetric quantification, and definition of complex structures and anatomy.
Tissue Characterization
Myocardial edema
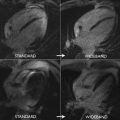
CMR has the advantage to offer information on myocardial tissue remodeling complementary to traditional morphologic and functional measurements. Compelling evidence indicates that myocardial edema, inflammation, abnormal strain, and expansion of interstitial fibrosis occur before cardiac dysfunction develops. Indeed, experimental and clinical observation have shown that a multiple-parametric CMR protocol, incorporating recently developed T1 and T2 mapping techniques, may detect very early signs of myocardial tissue remodeling, improving the current understanding of chemotherapy-induced cardiotoxicity, facilitating future development and implementation of potential preventive treatments. Detection of myocardial edema and inflation, which are mostly based on increased ratio of T2-weighted signal intensity of myocardium normalized to skeletal muscle, has been successfully applied to ischemic and nonischemic cardiomyopathy. In a well-designed animal study, comprising baseline and post-doxorubicin multiple-parametric CMR examination, Farhad and colleagues 54 demonstrated that both myocardial edema and expansion of interstitial fibrosis occurred prematurely, having also a significant association with animal mortality rates. Using widely available black-blood T2-weighted sequences, Ferreira de Souza and colleagues observed a significant rise in myocardial T2-weighted signal intensity ratio to skeletal muscle early after a moderate cumulative dose of doxorubicin (total dose of 240 mg/m 2 ) ( Figs. 3 and 4 ). Also, data suggest that myocardial edema, assessed by myocardial T2-weighted signal intensity normalized to skeletal muscle has been shown to be significantly associated with decreased RV systolic function, 12 months after anthracycline and/or trastuzumab treatment. Various groups are currently investigating the role of novel T2 mapping sequences to serially detect myocardial edema after cancer therapy, but up to now, T2-weighted imaging has not been broadly studied to establish the usefulness of edema quantification to monitor patients after cancer therapy.


Myocardial fibrosis
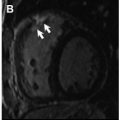
Although LGE imaging has been shown to precisely recognize both myocardial scar and replacement fibrosis, this technique may only accomplish partial assessment of the myocardial fibrosis burden. Because LGE imaging relies on relative signal intensity differences after gadolinium-based contrast administration, it can fail to identify interstitial myocardial fibrosis. In numerous reports, including retrospective and prospective studies, LGE was not uniformly detected after anthracycline-based chemotherapy. Actually, a negative LGE imaging study, frequently seen in anthracycline-induced cardiomyopathy ( Fig. 5 ), may not represent true absence of fibrosis, highlighting the limitation of LGE imaging in this setting. Although Fallah-Rad and colleagues showed evidence of subepicardial LGE in all patients who developed trastuzumab-induced cardiac dysfunction, several subsequent studies reveled conflicting results, with a report from Lawley and colleagues showing LGE in only 8% of the 25 women treated with trastuzumab. In addition, Neilan and colleagues also observed that LGE is an infrequent finding in patients treated with anthracycline-cardiomyopathy, occurring in only 6% of cases.