Despite recent advancements in newer biomarkers development and improved imaging techniques, the diagnosis of cardiac amyloidosis (CA) remains a frequent clinical challenge. In this setting, cardiac MR (CMR) imaging has emerged as a powerful tool to assess heart morphology and function, with the unique advantage of noninvasive tissue characterization. This article summarizes the CMR imaging common findings in CA and the latest research in this field, including delayed enhancement, native T1 mapping, and extracellular volume quantification.
Key points
- •
Cardiac MR imaging provides detailed assessment of cardiac function, volumes, and tissue characterization, thus it is very useful in the diagnostic pathway of cardiac amyloidosis (CA).
- •
Delayed enhancement detects myocardial infiltration secondary to amyloid deposition in patients with CA, and has both diagnostic and prognostic utility.
- •
Native T1 and extracellular volume calculation are recent mapping techniques that indirectly measure amyloid burden. These not only have high diagnostic accuracy for CA but are also strong independent predictors of outcomes.
Introduction
Amyloidosis is a multiorgan, infiltrative disease caused by abnormal protein deposition of insoluble amyloid fibrils on the extracellular matrix. There are a variety of different subtypes of amyloid disease, either hereditary or nonhereditary, each associated with specific precursor proteins, organ involvement, and prognosis.
Cardiac involvement plays an important role in patient prognosis and therapy decision. More than 95% of all cardiac amyloidoses (CAs) are related to immunoglobulin light-chain amyloidosis (AL) or transthyretin amyloidosis (ATTR). In AL-CA, which is related to multiple myeloma in up to 15% of cases, amyloid fibrils are derived from monoclonal immunoglobulin light-chains. AL-CA is associated with a poor prognosis, with a median survival after diagnosis not longer than 1 year. Transthyretin (TTR), the precursor protein in ATTR-CA, is a liver-derived plasma protein that acts as a transporter for thyroxine and retinol-binding protein. ATTR can occur either as a hereditary mutant (ATTRm) variant or as an acquired wild-type (ATTRwt) variant, the latter is also known as senile amyloidosis. ATTR is associated with a better prognosis compared with the AL-CA type, with a mean survival of 3 to 5 years for ATTRwt and about 2 years for the most common form of ATTRm in the United States (V122l mutation).
Despite recent efforts, both in terms of novel research and optimization of clinical management, and in the face of its increasingly recognized clinical relevance, amyloidosis remains an underdiagnosed disease for which the true incidence is still unknown. According to the most recent estimates, AL occurs in 6 to 10 per million individuals in the United States, whereas the most common variant of ATTRm (V122l mutation) is present in 3% to 4% of African Americans. ATTRwt (senile amyloidosis) is reported to be present in up to 25% of the elderly population on autopsy studies and in up to 13% of patients admitted with heart failure with preserved ejection fraction on a recent study using nuclear imaging techniques.
Novel specific and effective treatments for AL-CA and ATTR-CA are being increasingly reported, which has led to a growing interest in the early and accurate diagnosis of CA. Despite advancements in noninvasive cardiac imaging, with the recent introduction of echocardiographic speckle tracking techniques and nuclear imaging methods such as bone tracer scintigraphy, diagnosing CA is still challenging. This is particularly true for early-stage CA, in which serum biomarkers and electrocardiographic and echocardiographic findings might not yet be evident or typical for CA. In this context, cardiac MR (CMR) imaging has emerged as a valuable technique that is playing an increasingly important role in the assessment of CA.
The focus of this article is to summarize the state-of-the-art applications of CMR imaging for the diagnostic and prognostic assessment of patients with suspected or confirmed CA ( Table 1 ).
| Author, Year | Number of Subjects (amyloidosis) | Diagnostic Evaluation | Tissue Characterization Techniques Used | Prognostic Evaluation | Mean Follow-up (months) |
|---|---|---|---|---|---|
| Maceira et al, 2005 | 30 | — | DE | No | — |
| vanden Driesen et al, 2006 | 8 | — | DE | No | — |
| Perugini et al, 2006 | 21 (AL = 9; ATTR = 12) | — | DE | No | — |
| Vogelsberg et al, 2008 | 33 | SENS = 80%; SPEC = 94%; PPV = 92%; NPV = 85% | DE | No | — |
| Ruberg et al, 2009 | 28 (AL only) | SENS = 86%; SPEC = 86%; PPV = 95%; NPV = 67% | DE | Yes | 29 (range 5–36) |
| Austin et al, 2009 | 47 | SENS = 88%; SPEC = 90%; PPV = 88%; NPV = 90% | DE | Yes | 12 |
| Syed et al, 2010 | 120 (AL = 100, ATTR = 20) | — | DE | No | — |
| Banypersad et al, 2013 | 60 (AL only) | — | DE, native T1, ECV | No | — |
| Karamitsos et al, 2013 | 46 | SENS = 92%; SPEC = 91%; ACC = 92% | DE, native T1 | No | — |
| Dungu et al, 2014 | 97 (AL = 46, ATTR = 51) | — | DE | No | — |
| Pozo et al, 2014 | 130 | SENS = 67%; SPEC = 86%; ACC = 88% | DE | No | — |
| White et al, 2014 | 90 | SENS = 93%; SPEC = 70%; ACC = 84% | DE, TI scout | Yes | 29 (IQR: 12–44) |
| Fontana et al, 2014 | 164 (AL = 79, ATTR = 85) | AUC = 0.85 (0.79–0.92) | DE, native T1 | No | — |
| Banypersad et al, 2015 | 100 (AL only) | — | DE, native T1, ECV | Yes | 23 (IQR: 6–25) |
| Fontana et al, 2015 | 250 (AL = 119, ATTR = 122) | — | DE, native T1, ECV | Yes | 24 ± 13 |
| Martinez-Naharro et al, 2017 | 342 (AL = 50, ATTR = 292) | — | DE, native T1, ECV | Yes | 19 ± 14 |
| Martinez-Naharro et al, 2018 | 227 (ATTR only) | AUC = 0.91 (0.87–0.94) | DE, native T1, ECV | Yes | 32 ± 17 |
Morphologic Features
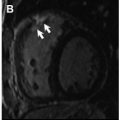
Cardiac amyloidosis is characterized by the infiltration and deposition of amyloid fibrils in the interstitial space of the myocardial tissue, resulting in progressive cardiac wall thickening and diastolic dysfunction, and eventually leading to restrictive cardiomyopathy.
Endomyocardial biopsy (EMB) is the gold standard for the diagnosis of CA. However, EMB is an invasive technique, limited by local expertise, and not widely available. Therefore, current diagnostic strategies are frequently based on clinical, laboratory, and imaging findings, often associated with a noncardiac biopsy.
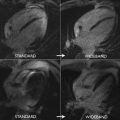
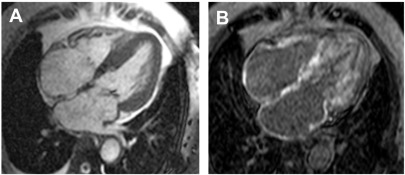
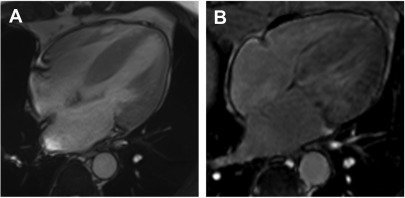
In the past decade, CMR imaging has emerged as a powerful imaging technique that provides detailed assessment of the classic features present in the later stages of CA ( Box 1 ), including left ventricular hypertrophy (LVH), disproportionate atria enlargement, and increased atrial wall thickness ( Fig. 1 ).
- •
Decreased LV end-diastolic volume (100–131 mL; 58–69 mL/m 2 ), leading to reduced stroke volume (59–72 mL; 32–38 mL/m 2 ) despite a preserved or mildly reduced LV ejection fraction (LVEF) ranging from 56% to 60%. Importantly, LVEF may be reduced in endstage disease.
- •
Left ventricle hypertrophy (LVH), either concentric or asymmetric (more likely asymmetric in ATTR [79%] and more likely concentric in AL [68%]).
- •
Right ventricle thickening is also suggestive of cardiac amyloidosis.
- •
Disproportionate atria enlargement with increased atrial septal thickness due to amyloid deposition within the atrial walls (a nonspecific finding).
- •
Pericardial (39%–52%) and pleural (41%–47%) effusions may be present as a nonspecific finding.
- •
Thickening of at least 1 cardiac valve, associated with mild to moderate regurgitation in up to 85% of patients (a nonspecific finding).
Left Ventricle Volumes and Systolic Function
Typically, the left ventricle (LV) is not dilated in up to 80% of patients with CA on autopsy studies. In vivo, LV end-diastolic volume (LVEDV) is usually reduced. This frequently leads to a reduced LV stroke volume (LVSV) despite preserved or only mildly reduced LV ejection fraction (LVEF). This pattern has been reported in several prior studies, with the mean LVEDV ranging from 100 to 131 mL (58–69 mL/m 2 ), LVSV from 59 to 72 mL (32–38 mL/m 2 ), and LVEF from 56% to 60%. However, importantly, LVEF can be severely compromised in cases of advanced CA.
Left Ventricle Hypertrophy
Although concentric LVH is regarded as a hallmark of CA, several studies have shown that alternative remodeling patterns may be present. Pozo and colleagues reported that subjects with CA often have increased LV mass and wall thickness, with concentric LVH present in 58%, whereas alternative patterns are present in 42% (18% eccentric LVH, 16% concentric remodeling, and 8% normal geometry). Interestingly, in the same study, the investigators showed that the region of maximal LV wall thickness, which is located in the basal anteroseptum in 99% of patients without CA, was found in other LV wall locations in 33% of subjects with confirmed CA.
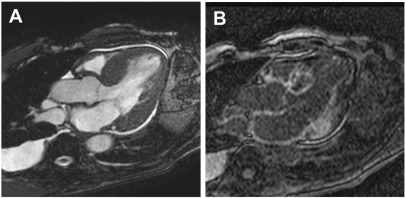
Recently, Martinez-Naharro and colleagues reported asymmetrical septal LVH to be the most common pattern of LV remodeling in ATTR-CA (79% of subjects), whereas concentric LVH was more common on AL-CA (68% of subjects). The 2 main subtypes of asymmetrical septal hypertrophy were seen in 55% (sigmoid septum) and 24% (reverse septal contour) of subjects with ATTR, the latter also being considered a typical finding in hypertrophic cardiomyopathy ( Figs. 2 and 3 ). Asymmetrical LVH was seen in only 14% of subjects with AL-CA and all of them had the sigmoid septal pattern. No LVH was seen in 3% of subjects with AL-CA and in 18% of the subjects with ATTR-CA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree