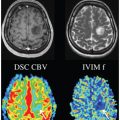
Assessment of Liver Tumors with IVIM Diffusion-Weighted Imaging
Intravoxel incoherent motion diffusion-weighted imaging (IVIM DWI) is a noninvasive imaging method that probes the mobility of microscopic water molecules in biologic tissues and incorporates the contributions of microvascular perfusion and true cellular diffusion restriction. This method is well suited for investigation of focal liver lesions (FLL). Important technical considerations for IVIM include the selection of b values, acquisition technique, field strength, fitting algorithm, and postprocessing methods. Recent studies have explored the use of IVIM DWI for a variety of applications, including characterization of liver lesions, prediction of tumor grade, and evaluation of the post-treatment response. This chapter will discuss in detail the promising early results of IVIM assessment of FLL.
11.1 Introduction
Given the increasing utilization of magnetic resonance imaging (MRI) and recent technological advances in hardware and software, FLL are being detected with increasing frequency in the clinical setting. Accurate detection and characterization is essential for diagnosis and appropriate management; and the superior soft-tissue contrast resolution of MRI is well suited for this purpose. There is increasing interest in the development and validation of advanced MRI methods to provide both anatomic and functional information. DWI has shown its value for a variety of clinical indications and is now widely adopted in most clinical abdominal protocols. IVIM has been recently implemented predominantly in the research setting, given the ability of this method to noninvasively and simultaneously probe several physiologic processes affecting tissues. An additional benefit of the use of DWI methods is the lack of intravenous gadolinium– based contrast administration, which is of increasing concern in the era of nephrogenic systemic fibrosis (NSF) [1] and given recent reports of gadolinium deposition in the central nervous system [2]. In this chapter, we will explore the use of the IVIM DWI technique in FLL, with an emphasis on technique, clinical applications, limitations, and future directions.
11.2 Background on Focal Liver Lesions
FLL are generally either benign or malignant, with rare premalignant lesions (hepatocellular adenomas [HCAs] and high-grade dysplastic nodules in cirrhosis). The most commonly encountered benign liver lesions are hemangiomas (reported prevalence 0.1%–20.0%), cysts (reported prevalence 0.06%–17.8%), focal nodular hyperplasia (FNH) (reported prevalence 0.8%–3.2%), and HCA (reported prevalence 0.4%–1.5%) [3]. The most frequently encountered malignant lesions are primary liver cancers, such as hepatocellular carcinoma (HCC) (reported incidence of 4.8 per 100,000/year), intrahepatic cholangiocarcinoma (ICC) (reported incidence of 1.6 per 100,000/year), and combined “bi-phenotypic” hepatocellular- cholangiocarcinoma (cHCC-ICC) [4]. The age-adjusted incidence of cHCC-ICC is not well known, as these lesions were only recently recognized by the World Health Organization (WHO) as a distinct subtype of ICC. However, they represent approximately 0.4%–14.5% of all primary liver cancers [5, 6]. Secondary (metastatic) cancers from a variety of primary cancer sites occur approximately 18 times more frequently than primary liver cancers [7]. It is essential to avoid unnecessary biopsy in patients with benign lesions, given the invasive nature and risk of complications associated with tissue sampling [8, 9]; and it is necessary to accurately diagnose malignant lesions for disease staging and directing patient management.
HCC is the sixth-most-common form of cancer and the third leading cause of cancer-related death [10]. HCC is the most common primary liver cancer and is closely linked to the presence of chronic liver disease, fibrosis, and cirrhosis, with an annual incidence ranging from 0.25% to 7.6%, depending on the etiology of the underlying liver disease and other risk factors [11]. HCC has high morbidity and mortality, with a five-year survival of <10% when detected after symptoms develop [12]. Accurate diagnosis is especially important as HCC is typically an imaging diagnosis, and tissue confirmation is only performed in a limited number of cases that demonstrate an atypical imaging appearance [13]. Depending on the tumor stage and the severity of the background liver disease, patients with HCC are generally treated with resection, liver transplantation, locoregional therapy (LRT), or systemic therapy using novel molecular-targeted agents [14]. Although arising less commonly, ICC and cHCC-ICC share common risk factors with HCC but will differ in both treatment strategy and prognosis [6, 15]. Currently, ICC and cHCC-ICC are considered relative contraindications to liver transplant and are also more sensitive to systemic chemotherapy, whereas systemic chemotherapy has not been shown to be effective for HCC [6, 16]. Therefore, it is essential that benign and malignant liver lesions are accurately detected and characterized, in order to inform management decisions.
11.3 Current Evidence on the Use of DWI and ADC for Liver Tumor Detection and Characterization
Multiphasic contrast-enhanced MRI (CEMRI) is currently the clinical standard for the assessment of FLL [17]. However, there is increasing interest in the validation of noninvasive and noncontrast methods, including DWI, for the evaluation of liver lesions. There is evidence of the potential added value of DWI for the detection and characterization of FLL. Low-b-value (b<100) DWI produces “black blood” images due to a suppressed vascular flow, leading to improved conspicuity of lesions, particularly for lesions adjacent to blood vessels [17].
11.3.1 DWI for Liver Lesion Detection
The diagnostic performance of DWI in liver lesion detection has been quite extensively compared with that of conventional sequences, such as T2-weighted imaging (T2WI) and CEMRI. Several studies have shown that DWI outperforms T2WI in terms of detection of FLL, including liver metastases, with accuracy values of 84.4%–92% reported for DWI versus 47%–70.1% reported for T2WI [18–20]. The detection rate of HCC in particular has also shown to be significantly higher using DWI compared to T2WI (80.5% vs. 53.9%) [19]. With respect to comparison with CEMRI, a recent meta-analysis by Vilgrain et al., which included 39 published articles with a total of 1989 patients, reported sensitivity values of 87.1%, 90.6%, and 95.5% for the detection of hepatic metastases using DWI, gadoxetic acid–enhanced MRI, and the combination of both methods, respectively. While DWI performed significantly worse than gadoxetic acid–enhanced MRI for lesion detection (P = 0.0001), it was shown that the combination of DWI with gadoxetic acid– enhanced MRI led to significantly better lesion detection compared to gadoxetic acid–enhanced MRI alone (P < 0.0001) [21]. For HCC, reported results on comparison between CEMRI and DWI have been mixed. Piana et al. showed improved sensitivity for HCC detection using DWI compared to CEMRI in 91 patients with 109 HCC lesions (72.5%–81.7% vs. 59.6%) [22], while Park et al. showed that DWI was outperformed by CEMRI (75.8% vs. 87.9%) for HCC detection in 52 patients with 72 HCC lesions (using explant correlation) [23]. Another study reported that combined DWI and CEMRI yielded improved sensitivity (95.2%) for the detection of small HCC lesions (<2 cm) in a cohort of 130 patients [24]. It is likely that the presence of background liver fibrosis and cirrhosis, which commonly coexist with HCC, contributes to lower tumor detection rates [25]. Reports of DWI for the detection of ICC are limited. A study by Park et al. showed that target appearance on DWI (a hyperintense rim with a central hypointense area) was the only significant predictor of ICC versus HCC among other features based on gadoxetic acid–enhanced MRI and DWI [26]. This study indicates the potential value of DWI for the detection of ICC, although this needs to be validated in a separate study.
11.3.2 DWI for Lesion Characterization
The apparent diffusion coefficient (ADC) measured with DWI has been extensively investigated as a parameter to differentiate between types of FLL. Several studies have assessed the diagnostic performance of ADC for differentiation between benign and malignant liver lesions. Using ADC cutoff values of 1.5–1.6 × 10–3 mm2/s, sensitivity values of 63.5%–87% and specificity values of 77.3%–90% have been reported for the diagnosis of malignant liver lesions [19,27–29]. In a large DWI study involving 542 FLL in 382 patients, it was found that cysts could be easily distinguished from other lesions but that there was significant overlap between ADC values of benign and malignant liver lesions [28]. Recent studies for HCC, ICC, and metastatic disease have also demonstrated the value of ADC measurement in predicting tumor grade, with an inverse relationship of ADC with increasing tumor grade [30–32].
In summary, DWI is increasingly being employed for the detection and characterization of liver lesions. While results have been promising, DWI cannot be used as a standalone technique for liver cancer imaging because of the overall lower detection rate of hepatic lesions compared to CEMRI and the substantial overlap between ADC values of benign and malignant lesions. More advanced DWI methods, including IVIM DWI, may potentially improve the sensitivity of DWI to liver lesion malignancy.
11.4 IVIM DWI for the Assessment of LiverLesions: Technical Considerations
Because IVIM incorporates the contributions of microvascular perfusion and true cellular diffusion restriction, it has attracted much attention for the assessment of liver lesions. However, IVIM DWI for the detection and characterization of liver lesions is challenging, given the generally long acquisition times and respiratory motion. IVIM parameter quantification may be affected by several factors, including b values, acquisition scheme, field strength, and processing methods. Several technical considerations need to be taken into account to improve the accuracy of IVIM DWI for the imaging of liver lesions and are discussed in detail below.
11.4.1 Acquisition
Selection of b values
IVIM DWI employs a biexponential fitting model to extract three different variables from multi-b-value DWI data: pseudodiffusion coefficient D* (or D fast), (true) diffusion coefficient D (or D t), and perfusion fraction (f or PF). A detailed description of the IVIM DWI technique can be found in Chapter 1, “Introduction to IVIM MRI.” Due to the fitting of a larger number of variables compared to monoexponential fitting, the IVIM model is overall less stable than the monoexponential diffusion model that is used to extract ADC. While in theory four b values would be sufficient to fit the three IVIM parameters, the use of more b values improves parameter estimation. Dyvorne et al. showed that the use of an optimal set of four b values (0, 15, 150, and 800 s/mm2) led to estimation errors that were below the test-retest repeatability of IVIM parameters in the liver [33]. Ter Voert et al. found that the use of 16 b values ranging from 0 to 1000 s/mm2 yielded accurate results of IVIM for the characterization of liver lesions [34]. However, in light of restricted acquisition time in clinical applications, using at least 11 b values may be sufficient since the largest gain in error reduction was seen when moving from 4 to 11 b values [34].
Signal-to-noise ratio
DWI images with a sufficiently high signal-to-noise ratio (SNR) need to be obtained in order to allow for reliable fitting of IVIM DWI parameters. Using Monte Carlo simulations it was found that the accuracy of IVIM parameter estimation depends strongly and nonlinearly on the SNR and that an SNR value of at least 8 is required for IVIM DWI of tissues with high IVIM perfusion, such as the liver [35]. For low-perfused tissues, an SNR of at least 40 has been recommended [36]. Low perfusion may occur in tumor tissues, particularly when (treatment induced) necrosis is present. Therefore, an SNR of at least 40 would be advisable for reliable IVIM DWI of liver lesions.
Acquisition scheme
Published studies on IVIM for the evaluation of FLL have used a mix of respiratory-triggered (RT) [34,37–50], free-breathing (FB) [51–57], and breath-hold [58–60] DWI techniques for IVIM acquisition. However, reports on the effect of the breathing scheme on IVIM DWI parameters in hepatic lesions are scarce. In a study of 30 patients with 37 small HCC lesions (range 0.5–2.0 cm), it was found that, while image quality with RT IVIM DWI was superior to FB IVIM DWI (P = 0.02), IVIM DWI parameters in HCC lesions were not significantly different between the acquisitions (P > 0.24) [61]. These results indicate that RT IVIM DWI is not advantageous for the quantification of IVIM DWI parameters in HCC except for a better image quality. Since FB acquisition schemes generally allow for faster IVIM acquisitions, FB IVIM DWI may be the best option in time-constrained clinical applications of IVIM DWI of liver lesions.
Field strength
While it has been reported that IVIM DWI parameters vary significantly between MR scanners in abdominal organs, including liver [62], the effect of field strength as a single variable on IVIM DWI parameter estimation in the liver has been scarcely studied. In a study of 19 subjects, it was found that all liver IVIM parameters were significantly different between 1.5T and 3.0T, except for the ADC (P = 0.748) [63]. While the use of 3.0T for IVIM DWI would appear advantageous due to an improved SNR compared to 1.5T, 3.0T IVIM DWI images may be affected by increased magnetic susceptibility artifacts and eddy current–related distortions [64]. No studies have assessed differences in image quality and IVIM DWI parameters when using field strength of different values for hepatic lesions. Therefore, further investigation is warranted to determine which field strength is most suitable for IVIM DWI of hepatic lesions.
11.4.2 Postprocessing
Fitting algorithms
Since the accuracy of IVIM parameter estimation is strongly dependent on factors such as image quality and SNR, IVIM analysis requires a robust fitting method. Least-squares optimization is the mostly used fitting algorithm for IVIM modeling. However, it has been shown that Bayesian methods yield more stable and accurate IVIM parameters than least-squares methods [65, 66]. Unlike the least-squares method, which minimizes error residuals, the Bayesian methods determine the uncertainty of each parameter in the model and use a priori distributions of the IVIM parameters to determine joint posterior probability over all parameters. Superior fitting quality, as measured by variability and uncertainty estimates, of Bayesian versus least-squares methods has been demonstrated in the liver tissue of 20 patients with colorectal liver metastases [65]. While both fitting methods have not yet been compared in liver tumor tissue, it is expected that Bayesian modeling of IVIM data in liver tumors provides more stable and accurate parameter estimation. Nevertheless, only a few studies that used IVIM modeling of liver tumors have employed Bayesian modeling for the analysis of the IVIM data [40, 53]. Widespread implementation of Bayesian fitting algorithms for IVIM fitting may therefore be of value to improve parameter accuracy and stability.
Region of interest vs. parametric mapping
While most studies employed parametric mapping for IVIM analysis of liver lesions, several studies have used analysis of mean signal intensities in regions of interest (ROIs) within the lesion [39,40,50,52,53,55,58]. ROI-based analysis may be favorable in SNR-limited IVIM DWI acquisitions as it increases the SNR for IVIM parameter fitting [52]. However, care must be taken using this approach for the analysis of heterogeneous or cystic/necrotic tumors, for which the averaged ROI signal will contain diffusion signals from different tissue types with distinct diffusion properties. The observed biexponential diffusion behavior in such cases may arise from the presence of tissue components with distinct diffusion coefficients rather than from pure diffusion and pseudodiffusion effects, leading to incorrect IVIM parameter estimates.
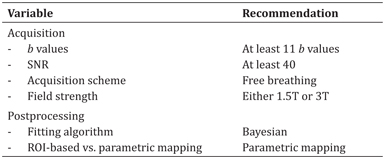
A summary of recommendations based on technical considerations for acquisition and postprocessing of IVIM DWI of liver lesions can be found in Table 11.1.
Table 11.1 Recommendations for acquisition and postprocessing of IVIM DWI data of liver lesions
11.4.3 Quality Control
Reproducibility and repeatability of IVIM parameters
Several studies have assessed IVIM parameter measurement repeatability and reproducibility. Grouped results for reported coefficient of variations (CVs) for test-retest repeatability of IVIM parameters for FLL have shown that D* and PF demonstrate poor measurement repeatability whereas the ADC and D generally show acceptable repeatability: ADC (8.3%–27.36%), D (8.7%–27.99%), D* (11.9%–73.84%), and PF (13.7%–43.41%) [40,42,53,67].
Interestingly, Kakite et al. showed that IVIM parameter repeatability was generally better for HCCs located in the right lobe compared to those in the left lobe [53]. With respect to parameter reproducibility, a recent study has assessed the reproducibility of IVIM parameters in HCC and FNH by two different 1.5T scanners [58]. While no significant difference in the measurement values of PF and D was observed, D* for HCC and ADC for FNH were significantly different between the two MRIs (both P = 0.02) [58]. Results from these studies indicate that D* suffers from the weakest repeatability and reproducibility, which could be secondary to a combination of physiologic and hemodynamic variability and technical limitations inherent to IVIM.
11.5 Applications of IVIM for the Assessment of FLL
11.5.1 Characterization of Benign and Malignant Liver Lesions
Early studies have shown mixed results regarding the performance of IVIM parameters for making the distinction between solid benign versus malignant liver lesions (Figs. 11.1–11.3). Malignant lesions are in theory well suited for investigation using IVIM, given the more complicated tissue microstructure, increased tumor cellularity, decreased extracellular space, accumulation of macromolecules, and altered intratumoral vascularity/perfusion. Grouped results of average IVIM parameter values for benign and malignant lesions from prior studies are summarized in Table 11.2. Several studies have shown that D (reflecting true diffusion restriction) is significantly lower in malignant lesions compared to benign lesions [42,43,50,51,55]. Results for the perfusion parameters PF and D* have been mixed for the same studies. Furthermore, the added value of IVIM parameters compared to standard monoexponential ADC has also been mixed, with superior performance of ADC over IVIM parameters shown in some studies [51, 55]. Overall, the area under the curve (AUC) to accurately discriminate malignant from benign lesions ranges from 0.75 to 0.971 for D and from 0.75 to 0.983 for ADC [42,43,50,51,55,57,58].
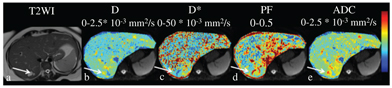
Figure 11.1 A 52-year-old female with NASH fibrosis (stage 3), steatosis, and a benign liver lesion. A 2.7 cm segment 7 hemangioma was identified at MRI with T2 and IVIM DWI performed with b values of 0–800 (arrows). D/D*/PF/ ADC values for the lesion were 1.4/17.6/0.27/1.7. ADC, D, and D* values were expressed as 10–3 mm2/s, and the PF is expressed as a fraction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree