Grade 1
Grade 2
Grade 3
Grade 4
Grade 5
Fatigue
Fatigue relieved by rest
Fatigue not relieved by rest; limiting instrumental ADL
Fatigue not relieved by rest, limiting self care ADL
−
−
Although asthenia represents a frequent clinical occurrence among cancer patients, its actual prevalence remains to be defined. It is estimated that 4–99 % of cancer patients experience asthenia during the course of their illness [6–8]. The wide range of this estimate could be attributed to the heterogeneity of the epidemiological studies (study population, asthenia definition, methods used to quantify fatigue) from which these data were derived.
In particular, asthenia rates are higher among patients receiving active treatment. Stashi et al. reported that while 50–75 % of patients presented at the time of diagnosis with asthenia, a higher rate experienced asthenia during chemotherapy (80–96 %) or radiotherapy (60–93 %) courses [9]. These rates remain high or even increase as patients with incurable disease progress [10]. Moreover, it seems that fatigue persists in a substantial proportion (~30 %) of patients rendered disease-free after completion of therapy (chronic fatigue) [11]. Indeed, a higher prevalence of persistent asthenia is reported in women surviving breast cancer compared to individuals without a history of cancer [12, 13].
Lower rates of asthenia are reported from studies adopting more explicit diagnostic criteria. According to a validation study in cancer survivors, although 37 % of patients reported fatigue, only 17 % of them met the proposed ICD-10 criteria for the diagnosis of CRF [11]. Contrarily, studies using telephone interviews reported higher prevalence of asthenia among cancer patients [14, 15]. In these trials fatigue was “defined” as a positive answer to the question: “Do you feel tired?”.
Taking into account its subjective nature, the unconformity between the reported rates of asthenia when patients, caregivers or oncologists are asked seems justifiable. Generally, caregivers report higher rates than patients. Although oncologists’ estimation of asthenia prevalence is even lower, they believe that this clinical syndrome is underdiagnosed [15].
Finally, patients with glioblastoma [16], lung cancer [17] and patients with bone metastases and compromised respiratory function due to extensive lung disease seem to exhibit a higher incidence of asthenia. The latter demonstrate the role of other symptoms (pain, dyspnea) in enhancing fatigue severity [10].
38.3 Pathophysiology
Beginning in the 1980s, efforts have been mounted to shed light on asthenias’ pathophysiology. It is now believed to be multifactorial, as inflammatory cytokines dictate the synergistic interactions between treatment, host and tumor mechanisms. Studies in humans and animal models provide the theoretical background of the proposed hypotheses. Overall, two major components of CRF have been recognized: (i) a central, involving dysregulation of serotonin neural-signaling pathways, hypothalamic-pituitary-adrenal (HPA) axis impairment, vagal-afferent signaling, circadian rhythm dysregulation and (ii) a peripheral, related with altered muscle metabolism (decreased ATP concentration and protein synthesis, increased lactate production). Increased inflammatory activity, reflected by high –plasma and tumor tissue- levels of cytokines, relies on the core of the above mentioned processes.
Inflammation has been recognized as a fundamental process in oncogenesis and tumor progression [18–20]. There is a growing amount of evidence showing a strong correlation between high levels of several mediators and biomarkers of inflammation with asthenia, both in patients with various tumor types [17, 21–23], as well as in cancer survivors [24–26]. In this chronic inflammatory response, the T-cell immunity plays a fundamental role. Bower et al. have shown that breast cancer survivors reporting persistent fatigue had significantly elevated CD4+ and CD56+ T-cell subpopulation compared to non-fatigued survivors [27]. In mice models, tumor progression was associated with depressive-like behaviors and fatigue even before any loss of muscle mass was documented. These alterations came together with an increase in IL-1β expression in the cortex and hippocampus of the mice [28]. In humans, it has been proposed that peripheral inflammation, generated by cancer itself or antineoplastic treatment, leads to production of various cytokines [29]. Pro-inflammatory cytokines, then, cross the blood-brain barrier and act on neural signaling of behavioral circuits in the central nervous system (CNS) that regulate emotion, cognition, motivation and vigilance [30]. The final result from the above described interaction is the emergence of certain symptoms that frequently co-occur [31]. These include asthenia, depression and sleep disturbances [32]. Specifically, IL-6 plasma levels have been positively correlated with fatigue, poor sleep quality and major depressive episodes in breast cancer and pancreatic cancer patients, respectively [33, 34]. Finally, it has been suggested that certain gene polymorphisms are associated with fatigue via regulation of pro-inflammatory cytokine production [35–38]. Nevertheless, these early findings require further validation in larger prospective trials [39].
In healthy individuals increased 5-HT levels [40] and up-regulation or increased sensitivity of 5HT-receptors in the hypothalamus [41] are associated with the development of fatigue after prolonged exercise. In cancer patients, cytokines such as TNFa or IL-1 are thought to enhance serotoninergic signaling in the CNS, as has been shown in cell lines and animal models [42–44].
The HPA axis normally regulates cortisol production. Fatigue has been linked with down-regulated HPA axis function and hypocortisolemia [45]. It is believed that pro-inflammatory cytokines in the context of cancer may disrupt HPA axis function via diminishing corticotropin-release hormone stimulation [46, 47]. In a study, women with breast cancer experiencing fatigue had lower serum cortisol levels than non-fatigued patients [48, 49]. However, other studies report an inverse relation between cortisol –or its metabolites- levels and fatigue [50, 51]. Moreover, various medical disorders such as sleep disturbance [52] and treatment modalities -e.g. radiotherapy, specific chemotherapy agents and glycocorticoids [53–55] – may directly influence HPA axis function, contributing to the emergence of CRF. As a conclusion, the connection between HPA axis dysregulation and asthenia remains unclear.
Circadian rhythm dysregulation has been implicated in the development of asthenia through two different pathways: altered patterns of endocrine organ function and sleep disorders. Several studies have found frequent circadian rhythm disruption in cancer patients, conferring a poor prognosis by inducing tumor progression [56, 57]. In particular, Bower et al. has reported rather flattened diurnal cortisol slope in breast cancer patients experiencing fatigue [58], while Weinrib et al. showed a strong association between nocturnal cortisol dysregulation and fatigue in ovarian cancer patients [59]. It is proposed that in the context of the systemic inflammatory response in cancer patients, TGFa promotes fatigue by dismantling the circadian axis through interaction with the epidermal growth factor receptor [60, 61]. Furthermore, fatigue positively correlates with sleep disorders such as restless sleep [62–64]. Particularly, in breast cancer patients disrupted sleep patterns were associated with flattened circadian rhythms and fatigue, irrespective of the presence of depression [65].
According to the vagal-afferent–activation hypothesis, pro-inflammatory cytokines and factors released from tumor tissue may act as neuro-modulating agents, stimulating vagal-afferent nerves. This activation causes a reduction in motor-muscle functional capacity [49] and promotes “sickness-behaviors” (e.g. depression, sleep disorders, fatigue, psychomotor slowing, anorexia) [30]. Several studies in animal models have provided evidence of a vagal reflex resulting in certain behavioral changes that enhance the debilitating sense of asthenia [66–68], probably via vagal nerve-mediated IL-1β production [69, 70]. In support of the latter, it has recently been shown that vagotomy reduces non-rapid eye movement sleep (NREMS) by undermining the TNFa-induced IL-1β production in the brain of mice [71]. However, it should be noted that this theory remains to be confirmed in humans. There is only indirect proof for increased vagal nerve activity in fatigued cancer patients [72].
ATP is the energy currency of human cells and the main source of energy for skeletal muscles. Asthenia, also described as lack of energy, is associated with a depletion of intracellular ATP stores. In tumor models, deformities in sarcoplasmic reticulum and mitochondria alter ATPs’ metabolic pathways in skeletal muscles, contributing to the energy deficit in cancer patients [73]. Asthenia is inextricably linked to cancer cachexia and its features. Thus, activation of non-profitable biochemical circles (e.g. Cori circle) and increased resting energy expenditure may multiply energy insufficiency in cancer patients. Fatigue mediated through ATP hypothesis is categorized as “physical” or” peripheral” fatigue [74].
38.4 Contributing Factors
Asthenia is frequently accompanied by several symptoms and conditions that contribute to its ontogenesis.
Anemia, a common consequence of cancer itself and its treatment, is recognized as a major contributor to fatigue [75] and its correction is associated with improvements in both fatigue and QOL [76]. However, in terminally-ill and bedridden patients, hemoglobin levels are not correlated with fatigue [77] and anemia is not considered a causative factor.
Cachexia and muscle wasting share common pathogenetic mechanisms with asthenia [78]. Cancer-cachexia is a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass resulting in strength deterioration and exercise intolerance [79], contributing to the “asthenic” phenotype of cancer patients [80].
Other, often treatable factors include hypothyroidism, sleep disorders, pain, depression and other comorbidities. When present, all these factors form a vicious circle that enhances the debilitating character of asthenia (Fig. 38.1). According to the NCCN practice guidelines, all cancer patients reporting fatigue should be assessed as per the presence of all the above [81].
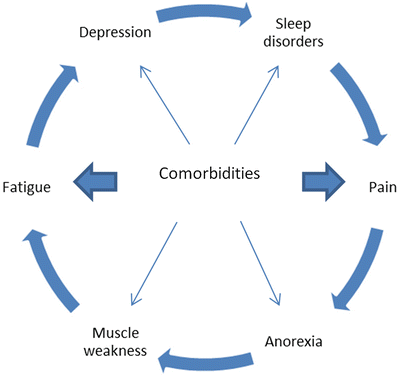

Fig. 38.1
Vicious circle of fatigue Comorbidities
Treatment related factors are also associated with asthenia. Radiotherapy can lead to decreased blood counts, diminished food intake, nausea and vomiting, diarrhea and impaired absorption of food nutrients as well as increased levels of plasma pro-inflammatory cytokines, contributing to the emergence or increasing the severity of already established fatigue [82, 83]. Chemotherapy has been, also, linked with fatigue via various pathways. Besides myelotoxicity, neurotoxicity, cardiotoxicity, gastrointestinal and direct CNS toxicity (intrathecal administration) [84, 85], chemotherapy can augment the development of cytokine-driven cognitive impairment [86]. Notably, different chemotherapy regimens induce different inflammatory responses [87]. Furthermore, hormonal changes related to certain treatment modalities in prostate [88] and breast cancer patients [89] are associated with fatigue. Moreover, interferon-α, a biologic response modifier, is known to cause fatigue and hypothyroidism in a substantial proportion of patients [90], while TKIs targeting the VEGF-receptor (sunitinib, sorafenib, pazopanib) are commonly related to fatigue development mostly via metabolic and gonadal, thyroid or adrenal function alterations [91].
Finally, medications used to alleviate symptoms in cancer patients such as opioids, antidepressants and certain anti-emetics (5-HT3 antagonists, NK 1-receptor antagonists) are commonly associated with fatigue [92]. Drug intake on an as-needed basis or switch to other drug categories with less sedative action are useful strategies towards minimalizing asthenia’s disabling impact on cancer patients.
38.5 Diagnosis and Evaluation
Albeit asthenia is an incapacitating symptom with severe effect on the patients’ QOL, it is often undiagnosed or underdiagnosed and sorely undertreated. Often patients do not report it, believing that it is an inevitable or incurable consequence of cancer, while others underrate this symptom due to fear that their treatment would change or even stop. Another major issue is the defective doctor-patient communication. Patients often complain for the shortage of time available with their physicians, while others don’t want to be criticized as a “moaner”. On the other hand, doctors underestimate the impact of asthenia on their patients’ daily life and don’t search for its presence. Even when patients report it, they decline fatigue as being an issue or encourage them to stoically accept it as an unavoidable and irremediable symptom of their illness [93, 94].
Hence, the first step in asthenias’ management should be the identification of patients suffering from it. In an effort to optimally define and distinguish CRF from other overlapping symptoms, a multidisciplinary group of cancer treatment and supportive care experts together with patient advocates developed certain diagnostic criteria (Table 38.2). These proposed criteria from the Fatigue Coalition [95] have been evaluated in various patient groups and proven to be a useful diagnostic tool with strong validity and reliability [11, 96, 97]. Indisputably, these criteria represent a solid cornerstone that is safe to build on towards development of a common, universal scientific language.
Table 38.2
Cancer-related fatigue: proposed diagnostic criteria
Proposed (1998 draft) ICD-10 criteria for cancer-related fatigue |
|---|
Six (or more) of the following symptoms have been present every day or nearly every day during the same 2-week period in the past month, and at least one of the symptoms is (A1) significant fatigue |
A1. Significant fatigue, diminished energy, or increased need to rest, disproportionate to any recent change in activity level |
A2. Complaints of generalized weakness or limb heaviness |
A3. Diminished concentration or attention |
A4. Decreased motivation or interest to engage in usual activities |
A5. Insomnia or hypersomnia |
A6. Experience of sleep as unrefreshing or nonrestorative |
A7. Perceived need to struggle to overcome inactivity |
A8. Marked emotional reactivity (eg, sadness, frustration, or irritability) to feeling fatigued |
A9. Difficulty completing daily tasks attributed to feeling fatigued |
A10. Perceived problems with short-term memory |
A11. Postexertional malaise lasting several hours |
B. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
C. There is evidence from the history, physical examination, or laboratory findings that the symptoms are a consequence of cancer or cancer therapy |
D. The symptoms are not primarily a consequence of comorbid psychiatric disorders such as major depression, somatization disorder, somatoform disorder, or delirium |
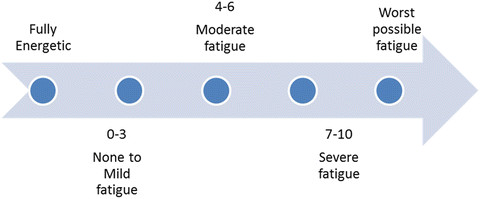
It should be emphasized that there is no general consensus on the target population, the optimal method or the frequency to screen for CRF. According to the NCCN guidelines, which in their majority were subsequently adapted by ASCO as well, all cancer patients should be screened, beginning at the time of diagnosis and then at regular intervals during antineoplastic treatment. Cancer survivors should also be screened for fatigue as clinically indicated, at least once yearly [98, 99]. Use of single-item tools has been proven brief and sensitive enough for identifying patients in need of a more focused evaluation [100]. Hence, patients are asked to rate their fatigue on a scale of 0–10 (Table 38.3). Patients reporting mild fatigue require counseling and re-evaluation at regular time intervals. General measures for fatigue management could be applied. Patients reporting moderate or severe fatigue should proceed to further assessment with a detailed history, a physical examination and possibly a targeted laboratory evaluation. The aim of this in-depth approach to the patient with asthenia is to recognize any treatable contributing factors and to delineate its impact on different aspects of the patients’ life (Table 38.4).
Table 38.3
Fatigue quantification, self-reported severity scale
Fatigue | |
|---|---|
Score | Severity |
0–3 | None to mild |
4–6 | Moderate |
7–10 | Severe |
Table 38.4
Focused evaluation of patients reporting moderate or severe fatigue
Component | Description |
|---|---|
History | Fatigue history: onset, time course, character, associations, relieving or exacerbating factors, impact on physical and cognitive capability, interference with ADL’s, social life and emotional status |
Review of systems: identify conditions and symptoms that can guide physical examination and subsequent laboratory testing | |
Personal history: smoking, alcohol abuse, activity level, employment history | |
Medication history: reveal contributing adverse effects or drug to drug interactions | |
Past medical history: already diagnosed conditions that may act as contributing factors | |
Social history: availability of caregiver support services | |
Evaluation of disease status | Determine disease burden, treatment type and response to therapy. Consider disease progression |
Address all potentially treatable contributing factors | All patients should be assessed for the presence of anemia, depression or anxiety, unrelieved pain, sleep disorders and other comorbidities such as hypothyroidism, adrenal insufficiency, active infection or cardiac, renal, hepatic, pulmonary, gastrointestinal and neurological dysfunction |
An initial laboratory work up should include complete blood count, a chemistry and electrolyte panel and TSH | |
Certain instruments could be used for pain or emotional distress assessment | |
If needed, consider referral to a relevant health care specialist | |
Nutritional assessment | Check for alterations in body weight and composition |
Evaluate the sufficiency of caloric intake | |
Check for fluid and electrolyte imbalances |
However, in order to receive a more comprehensive description of fatigues’ “burden” other tools can be applied. A systematic review of the published literature revealed 14 different scales broadly used in cancer patients that met their quality inclusion criteria. Among them, the EORTC QLQ C30 subscale, the FACT-F and the FQ were the best validated [101] (Table 38.5) [64, 102–108].
Table 38.5
Most important scales used for the measurement of cancer related fatigue
Instrument | Brief description |
|---|---|
Brief Fatigue Inventory (BFI) [102] | A nine item visual analog scale validated in various tumor types and in different languages |
Used primarily for the identification of patients suffering from severe fatigue. Unidimensional assessment tool | |
Functional assessment of cancer therapy-fatigue (FACT-F) subscale [103] | Part of FACT-G used to measure health–related quality of life (QOL) |
A 13 item scale validated in various settings | |
Useful for detecting minimal but clinical significant alterations over the course of time | |
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ C30) fatigue subscale [104] | Part of a 30 item QOL questionnaire |
A 3 item scale validated in various tumor types and multicultural settings | |
Easy to conduct | |
Useful mainly for the measurement of the physical dimension of fatigue | |
Inappropriate as the only measurement tool in terminally ill cancer patients | |
Fatigue Questionnaire (FQ) [105] | An 11 item scale |
Validated in various cancer types. Available comparative data between cancer patients and healthy controls | |
Easy to use | |
Evaluates physical and mental fatigues’ dimension | |
Can be used on a daily basis | |
A 12 item scale | |
Shorter than the 22 item revised Piper Fatigue Score, which is validated in breast cancer patients. A multidimensional tool, with limited supporting data | |
Cancer Fatigue Scale (Okuyama 2000) | A 15-item scale, capturing physical and psychological aspects of fatigue. Not validated in English language |
Multidimensional Fatigue Inventory (MFI-20) [107] | A 20 item scale |
Validated in various tumor types but on small study populations. Multidimensional tool | |
Can be time-consuming | |
Multidimensional Fatigue Symptom Inventory-short form (MFSI-30) [108] | Part of a more comprehensive 83 item screening tool |
A 30 item scale | |
Validated in various tumor types, mainly in breast cancer patients. Multidimensional tool | |
Can be time consuming | |
Limited data compared to other tools |
38.6 Treatment Strategies
On the grounds that asthenia is a multifactorial and multifaceted syndrome, our treatment approach should be multidisciplinary and multidimensional.
A team of healthcare professionals – including a physician, a nurse, a dietitian, a physiotherapist, a mental health professional and a social worker – should collaborate with the patient and his caregivers in order to create a supporting network with alleviating effect on the patients’ symptom burden. Following a general – common for all patients- approach, interventions for CRF could incorporate pharmacological or non-pharmacological measures as well as individualized treatment of an identified contributory factor.
38.6.1 General Measures
Educating patients and their families about CRF could be beneficial [109, 110]. Even before the occurrence of asthenia, they should be informed about the incidence, the potential causes, the hazardous impact in various aspects of their daily living and finally, the available general treatment strategies. The latter encompass various, chiefly shelf-applied modalities that could enhance one’s “defence” against fatigue development. Thus, energy conservation and activity management (ECAM), by giving priority to vital activities (e.g. hygiene) and postponing other less essential, can help patients regulate the usage of available energy resources [111]. Keeping diaries of the daily activities and of fatigue levels in certain time points can assist patients in scheduling their daily routine more efficiently. Additionally, a well-balanced diet ensuring a sufficient fluid, caloric, mineral and protein intake could also be beneficial.
Setting reasonable expectations, when confronting asthenia, is of paramount importance.
38.6.2 Treatment of Contributory Factors
All patients should be assessed for the presence of any treatable contributing factor (e.g., anemia, unrelieved pain, sleep disruption, or metabolic disorder). Upon identification, individualized therapeutic interventions should be applied as an initial approach to asthenia. Hence, anemia correction is associated with improvement in fatigue levels [76]. After declining other causes (e.g. blood loss, hemolysis), there are two options for anemia management: (i) red blood cell transfusions and (ii) use of erythropoiesis-stimulating agents in patients receiving chemotherapy [74] (Table 38.6). Furthermore, effective pain-control [112] and optimization of sleep disorders management [113] result in significant improvements in patient-reported fatigue.
Table 38.6
Risk and benefits of red blood cell transfusions and erythropoiesis-stimulating agent (ESA) use for the treatment of asthenia
RBC | ESA | |
|---|---|---|
Risks | Hypervolemia | Thromboembolic episodes (mainly when Hgb>12mg/dl) |
Acute transfusion reactions | Potentially adverse effect on patient clinical outcome. Not recommended for patients treated with curative intent | |
Viral infections | ||
Iron overload | ||
Benefits | Rapid increase in hemoglobin levels | Reduced need for transfusion |
Rapid clinical improvement | Gradual increase in hemoglobin levels |
38.6.3 Non-pharmacologic Interventions
Non-pharmacologic interventions may include exercise, cognitive-behavioral and psychosocial interventions, nutritional consultation and mind-body interventions [98, 99].
The role of physical exercise in alleviating fatigue both, in patients undergoing treatment and post-treatment survivors, is well established [114]. Although susceptible to various bias (lack of randomization, selection bias, heterogeneity in exercise delivery and tools used to measure outcomes), a growing body of evidence supports the use of exercise in reducing CRF [115]. Various exercise programs have been studied, including aerobic, resistance training or a combination, with duration ranging from 1,5 to 6 months and frequency ranging from 2 times/weekly to 2 times/daily. While resistance exercise improves physical strength, a Cochrane review reported that only aerobic training significantly reduces fatigue levels [116]. Efforts are mounted towards determination of the optimal intervention, as an ongoing trial is evaluating the relative benefit of low versus high intensity exercise [117]. ASCO endorses a weekly program of 150 min of moderate aerobic exercise (e.g. fast walking, cycling, or swimming) combined with two to three sessions of resistance training (e.g. weight lifting) for cancer survivors. However it should be noted that exercise programs should be tailored to the patients’ functional capacity and comorbidities. While walking programs are thought to be safe for most cancer survivors, those with severe fatigue, cardiomyopathy, neuropathy or other conditions interfering with exercise tolerance should be referred to the appropriate specialist [99]. Exercise interventions have also been proved beneficial in patients undergoing chemotherapy as well as hospitalized patients with advanced cancer [118]. Nevertheless, it is obvious that such individuals cannot follow the recommended exercise program. Advanced cancer patients exhibit a wide variety of barriers that interfere with their capacity to exercise. These include disease related (lytic bone metastases, respiratory insufficiency due to extensive lung disease) treatment related (anemia, neutropenia and avoidance of crowded places, severe thrombocytopenia and risk of hemorrhage) and patient related factors (shortage of time, reluctance, discouraging caregivers). These patients should be encouraged to participate in individualized, less intense training programs with a propitious effect on QOL [119, 120].
Psychological interventions are also effective management techniques. These include various modalities such as psychotherapy, psychosocial counseling, stress reduction and relaxation techniques, energy conservation and cognitive-behavioral interventions. Their aim, through group therapy or individual counselling, is to infuse cancer patients with self-monitoring and self-care strategies to better cope with fatigue [121]. Behavioral therapies assist patients to realize the effect of negative thoughts on their perceptions and daily routine [122]. Their goal is to improve patients’ functionality and self-dignity by manipulating the content of these thoughts. A review from the Cochrane database characterized these interventions as promising in CRF management, concluding that actions focused specifically on fatigue are more effective than nonspecific [123]. Although several randomized trials have proven psychological interventions efficacy in patients during treatment [124] and in cancer survivors [125], some patients seem not to benefit [126]. Further research is needed to better define the optimal intervention on a specific target population, in the context of asthenia management.
Mind-body interventions principally include mindfulness-based stress reduction (MBSR), hypnosis, music approaches and yoga. There are limited data from randomized trials that these approaches, alone or in combination with other, may reduce fatigue in cancer survivors [122, 127, 128] and this benefit seem to be long-lasting (~6 months). Nevertheless, the role of other modalities such as acupuncture and moxibustion is equivocal. Although a handful of clinical trials report a positive effect [129, 130] the authors of a recent systematic review [131] concluded that the available data are not sufficient enough to draw a definite conclusion. Pilot studies have also suggested that Reiki [132] or even medical Qigong [133] may be beneficial. More high-quality randomized trials are needed to elucidate their role in asthenia management.
Finally, nutritional support by encouraging a balanced-diet with weight and body composition monitoring may be considered as an integral part of fatigue management [134]. Increased intake of green-leafy vegetables and tomatoes as well as a diet rich in whole grain and antioxidant nutrients has been linked with lower fatigue levels [135]. Referral to a dietician may be appropriate.
38.6.4 Pharmacological Interventions
Conflicting to the respectable amount of data regarding non-pharmacological approaches for CRF management, pharmacological interventions have not been meticulously studied in controlled trials.
However, various agents have been tested with inconsistent results throughout the heterogeneous trials [136]. The most extensively evaluated drug-classes are psychostimulants and other wakefulness-promoting agents, antidepressants and steroids.
From all the above mentioned agents, the authors of a recent systematic review [136] concluded that, only methylphenidate – a CNS stimulant – is associated with a moderate but significant (p = 0.005) beneficial effect. Patients with more advanced disease and/or experiencing severe fatigue derived the most benefit [137]. Prolonged-treatment seems to display superior results compared to shorter duration programs with minimal side-effects, mainly vertigo and nausea [138]. Dexmethylphenidate and modafinil have also been linked with fatigue improvement compared to placebo. However, dexmethylphenidate resulted in a relatively high rate of drug-related adverse events [139], while modafinil probably only benefits patients with increased fatigue levels at baseline [140]. A therapeutic trial of psychostimulants should be undertaken in all patients, upon exclusion of other fatigue causes [98, 99].
In CRF-mouse models selective serotonin reuptake inhibitors (SSRI’s) have been shown to improve depressive-like behaviors but not fatigue [141]. Correspondingly, a Cochrane review didn’t document any benefit from these agents in ‘fatigued’ cancer patients [136]. Nevertheless, SSRI’s have proven their efficacy in the management of depression and sleep disorders in patients receiving antineoplastic treatment [142, 143]. Contrarily, in small studies bupropion –an atypical antidepressant- has been linked with lower CRF levels [144, 145]. Larger, placebo-controlled studies are needed to clarify its role in fatigue management. Presently, antidepressants are not recommended in asthenia management [98].
Steroids have been used for alleviation of various symptoms in incurable cancer patients. Although their exact role in this setting is still controversial [146], low-dose steroids are widely accepted as valuable options in palliative care [147, 148]. Nonetheless, two recent studies reported that a short course of steroids (dexamethasone and methylprednisolone) was associated with significant improvement in fatigue scores compared to placebo [149, 150]. Unless contraindicated, a trial of steroids should be considered [98] in advanced cancer patients.
Moreover supplements such as American ginseng and guarana may reduce fatigue in patients undergoing chemotherapy without additional toxicity [151, 152]. However, ambiguous interactions between ginseng and other drugs interfering with CYP3A4 could be a serious hindrance to its use [153].
Finally, other agents such as donezepil [154], multivitamins [155], L-carnitine [156, 157], coenzyme Q10 [158], infliximab [159], etanercept [160] and thyrotropin-releasing hormone [161] have also been evaluated for their efficacy against CRF. However, results are subjected to various biases (small samples, non-randomized or open-label studies) and must be interpreted with caution. Randomized controlled trials are needed to bridge the specific gaps in the current knowledge.
References
1.
Laird BJ, Scott AC, Colvin LA, McKeon AL, Murray GD, Fearon KC, Fallon MT (2011) Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage 42(1):1–11. doi:10.1016/j.jpainsymman.2010.10.261 PubMed