In this chapter, we will discuss the basic principles behind the physics of
magnetic resonance imaging (
MRI). Some of these principles are explained using
Newtonian physics, and some using
quantum mechanics, whichever can convey the message more clearly. Although this might be confusing at times, it seems to be unavoidable. In any case, we’ll try to keep it straightforward.
Electromagnetic Waves
To understand
MRI, we first need to understand what an electromagnetic wave is.
Table 2-1 demonstrates the characteristics of a variety of electromagnetic waves, including X-ray, visible light, microwaves, and radio waves. All electromagnetic waves have certain fundamental properties in common:
Table 2-2 summarizes the important electromagnetic windows in nature. In this table, the following notations are used:
keV = 103 eV = kilo-electron-volts
MeV = 10-3 eV = million electron volts
In
MRI, we deal with much lower energies than X-ray or even visible light. We also deal with much lower frequencies. (The energy of an electromagnetic wave is directly proportional to its frequency,
E =
hv.) The wave lengths are also much longer in the radio frequency (
RF) window.
Table 2-3 contains a few examples of frequency ranges in the electromagnetic spectrum.
This is why the electromagnetic pulse used in
MRI to get a signal is called an
RF pulse—it is in the
RF range. It belongs to the
RF window of the electromagnetic spectrum.
Spins and Electromagnetic Field
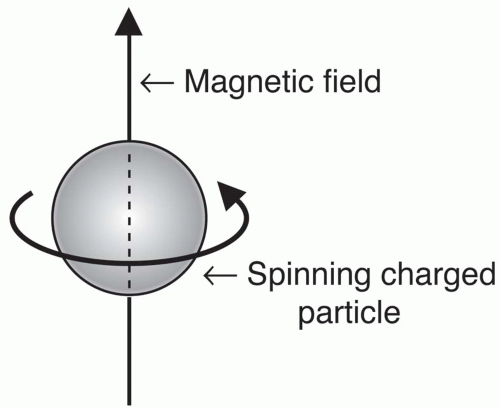
One of the pioneers of
NMR theory was Felix Bloch of Stanford University, who won the Nobel Prize in 1946 for his theories. He theorized that any
spinning charged particle (like the hydrogen nucleus) creates an
electromagnetic field (
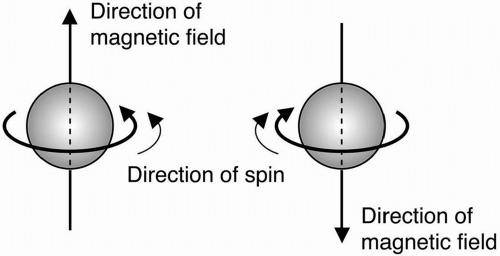
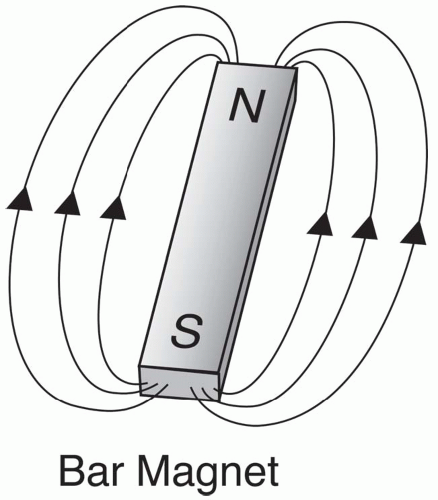
Fig. 2-3). The magnetic component of this field causes certain nuclei to act like a
bar magnet, that is, a magnetic field emanating from the south pole to the north pole (
Fig. 2-4).
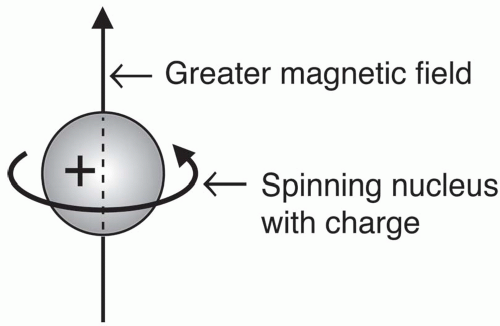
In
MRI, we are interested in
charged nuclei, like the
hydrogen nucleus, which is a single, positively charged proton (
Fig. 2-5).
The other thing that we know from quantum theory is that atomic nuclei each have specific energy levels related to a property called spin quantum number S. For example, the hydrogen nucleus (a single proton) has a spin quantum number S of 1/2:
S (1H) = 1/2
The number of energy states of a nucleus is determined by the formula:
Number of energy states = 2S + 1
For a proton with a spin S = 1/2, we have
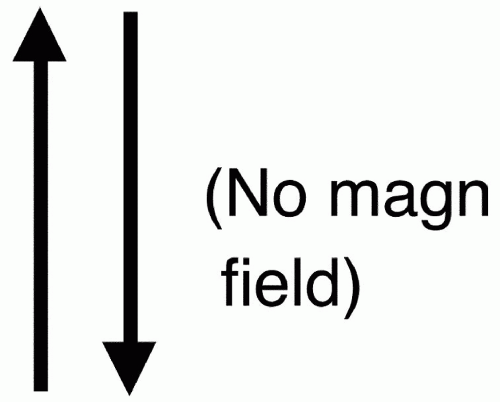
Number of energy states = 2 (1/2) + 1 = 1 + 1 = 2 Therefore, a hydrogen proton has two energy states denoted as − 1/2 and + 1/2. This means that the hydrogen protons are spinning about their axis and creating a magnetic field. Some hydrogen protons spin the opposite way and have a magnetic field in just the opposite direction. The pictorial representation of the direction of proton spins in
Figure 2-6 represents the two energy states of the hydrogen proton. Each one of these directions of spin has a different energy state.
Other nuclei have different numbers of energy states. For example, 13Na has a spin S = 3/2
Number of energy states of 13Na = 2 (3/2) + 1 = 4
The four energy states of 13Na are denoted as (−3/2, −1/2, 1/2, 3/2).
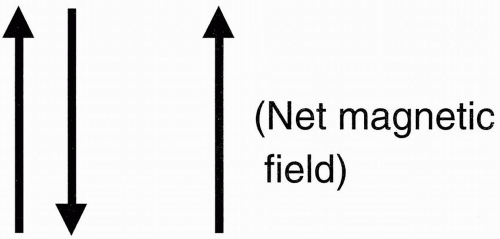
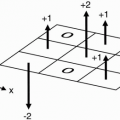
The important fact about all this is that in the hydrogen proton, we have one proton with two energy states that are aligned in opposite directions, one pointing north (parallel), and the other pointing south (antiparallel). (If there were an
even number of protons in the nucleus, then every proton would be
paired: for every proton spin with magnetic field pointing up, we’d have a paired proton spin with magnetic field pointing down (
Fig. 2-7). The magnetic fields of these paired protons would then cancel each other out, and the net magnetic field would be zero.) When there are an
odd number of protons, then there always
exists one proton that is
unpaired. That proton is pointing either north or south and gives a net magnetic field (
Fig. 2-8) or a
magnetic dipole moment (MDM) to the nucleus. Actually, an MDM is found in any nucleus with an
odd number of protons, neutrons, or both.
Dipole-dipole interactions refer to interactions between two protons or between a proton and an electron.
The nuclei of certain elements, such as hydrogen (
1H) and fluorine (
19F), have these properties (
Table 2-4). Every one of these nuclei with an
odd number of protons or neutrons can be used for imaging in
MR. However, there is a reason why we stay with hydrogen. We use
hydrogen for imaging because of its
abundance. Approximately 60% of the body is
water. We find hydrogen protons (1H), for example, in water (H2O) and fat (—CH
2—). Later on we’ll find out how we use the spin of the hydrogen proton and avoid the spins of all the other nuclei with odd numbers of protons.
Magnetic Susceptibility
All substances get magnetized to a degree when placed in a magnetic field. However, the degree of magnetization varies. The magnetic susceptibility of a substance (denoted by the Greek symbol χ) is a measure of how magnetized they get. In other words, χ is the measure of magnetizability of a substance.
To develop a mathematical relationship between the applied and induced magnetic fields, we first need to address the confusing issue regarding the differences between the two symbols encountered when dealing with magnetic fields: B and H. We caution the reader that the following discussion is merely a simplification; an advanced physics textbook will have details on the theory of electromagnetism. The field B is referred to as the magnetic induction field or magnetic flux density, which is the net magnetic field effect caused by an external magnetic field. The field H is referred to as the magnetic field intensity. These two magnetic fields are related by the following:
B = µH or µ = B/H
where µ represents the magnetic permeability, which is the ability of a substance to concentrate magnetic fields. The magnetic susceptibility χ is defined as the ratio of the induced magnetic field (M) to the applied magnetic field H:
M = χH or χ = M/H
Furthermore, χ and µ are related by the following:
µ = 1 + χ
making sure that the units used are consistent.
Three types of substances—each with a different magnetic susceptibility—are commonly dealt with in
MRI: paramagnetic, diamagnetic, and ferromagnetic. These are described below.