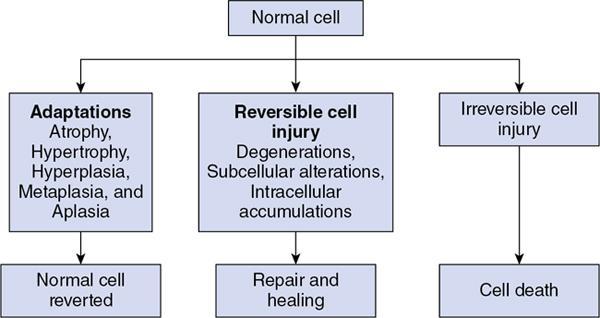
Indumathi Karnan, Geetha Devadas Cell injury is defined as the effect of a various stresses a cell encounters resulting in changes in its internal and external environment. There are various forms of cellular responses to cell injury as (Fig. 1.28.1) The common morphological changes in non-lethal reversible cell injury are These changes are termed as retrogressive changes. Reversible cell injury: The changes that happen within the cells are due to certain environmental changes and these changes are totally reversible when the stimulus is removed. Intracellular accumulations can occur with normal cell constituents like lipid, protein and carbohydrates or abnormal substances in the absence of certain enzymes or accumulation of pigments. Fatty liver is due to increased free fatty acids either from dietary metabolism or from the adipose tissues and leads to accumulation of triglycerides within the liver parenchyma as cytoplasmic vacuoles. Inflammation is the protective response of the living organism to the injury produced by any exogenous or endogenous agents. The agents can be infective agents like bacteria, virus, fungi, toxins and parasites or immunological agents like cell-mediated immunity and antigen antibody reaction or physical agents like heat, cold, radiation and trauma or chemical agents like inorganic or organic poisons or inert elements like foreign bodies. Though they are protective mechanisms, sometimes they are eligible of causing considerable damage to the body in setting up of the inflammation, e.g. anaphylaxis in insect bites. Inflammation is of two types: Acute when the response is short and is characterized by accumulation of fluids with activation of platelets and neutrophils, at times it can be so severe and called as fulminant acute inflammation. The other one is chronic inflammation that is characterized by the presence of chronic inflammatory cells such as lymphocytes, plasma cells and granulation tissue formation. The chronic active inflammation is the type of chronic inflammation in which there are acute exacerbations of disease activity. Granulomas are collection of epitheliod cells which are nothing but modified macrophages and are rimmed in the periphery by lymphoid cells and forms a circumscribed tiny inflammatory mass. It is an example of type IV hypersensitivity reaction. It starts as a protective defence mechanism by the host around the non-digestible antigen may be infectious particle like mycobacterium or non-infectious particle like suture material or any foreign body or even associated with autoimmune conditions. The evolution of granuloma Thus the granuloma is formed by collection of modified epitheliod cells in the centre and surrounded by T lymphocytes, multinucleated giant cells, healing fibroblasts or collagen depending upon the age of the granuloma (Fig. 1.28.9). The most common granulomatous inflammation in India is by Mycobacterium tuberculosis which presents with central caseation and well-formed epitheliod granulomas and other caseating granulomas are seen with tuberculoid type of leprosy. Non-caseating granulomas rae associated with a variety of bacterial infections as syphilis, donovanosis, brucellosis, cat scratch disease, tularemia, fungal infections like actinimycosis, blastomycosis, cryptococcosis, coccidiomycosis, parasitic infestations by schitosomiasis and non-infectious causes like sarcoidosis, crohn disease, silicosis and foreign body granuloma (Fig. 1.28.10). This test is performed by injecting 0.1 mL of purified protein derivative of Tuberculin protein as intradermal injection. Following that a delayed hypersensitivity develops and is identified as indurated area measuring more than 15 mm in 72 h, in individuals who are having or have been previously infected with tuberculous infection; patients with disseminated tuberculosis may not elicit an immune response as already they would have released a large amount of tuberculoproteins endogenously, masking the response to hypersensitivity test. A positive test is indicative of cell-mediated hypersensitivity but does not distinguish between disease and infection. Healing of skin wounds is a combination of regeneration and repair, It can by first intention (primary union) or second intention (secondary union). It happens in clean uninfected clear cut margins as surgically incised wounds without much loss of cells and tissue and in wounds were the edges are approximated. Here the space between the wounds are filled with haemorrhage and the site attracts the polymorphs and macrophages. The basal layer of the epithelium starts proliferating and migrating towards each other and along with the clot and inflammatory cells they form the scab. It gets organized by new collagen fibrils and fibroblasts proliferation and gives strength to the wound and by the end of 4 weeks are completely replaced with scar tissue (Fig. 1.28.11). Any wound with large tissue defect at times infected, having extensive loss of tissue and not approximated by surgical sutures heal by secondary union. Healing takes a longer process and results with ugly scars as compared to the primary union. Initial haemorrhage, inflammatory exudates and epithelial changes are similar to primary healing but they have florid fragile granulation tissue with neovascularization and wound contraction takes place by excess proliferation of myofibroblasta and hence leads to contractures. Following an injury the wound starts contracting after the initial inflammatory response and the contraction process is completed by 14th day. By the end of second week the wound is contracted to 80% of its original size. During the process there is proliferation of fibroblasts and myofibroblasts which gives structural support. The extracellular matrix has five components that include the collagen, adhesive glycoproteins which include fibronectin, tenascin and thrombospondin, basement membrane, elastic fibres and proteoglycans. Collagen is synthesized and secreted by ribosomes. Their synthesis is stimulated by various growth factors and degraded by collagenase. This balance is maintained by various local and systemic factors so that the normal content of collagen is maintained. When there is defective regulation of collagen synthesis it leads to hypertrophied scar, fibrosis and organ dysfunction (Fig. 1.28.12). Mechanisms of fibrosis Following a wound contraction there is excessive accumulation of collagen which is produced by fibroblasts leading to a permanent fibrotic scar Our human body has an internal environment that comprises of cells, tissue, plasma and interstitial fluid, all together maintain the normal functionality of the system. The mechanism by which this system is constantly maintained is called the homeostasis. Water: Water is the principle and essential constituent of the body. The total body water in a normal adult male comprises 60%–70% of the total body weight. Total body water is distributed into two compartments separated by membranes freely permeable to water. Electrolytes: The concentration of electrolytes are different in extracellular and intracellular compartments. In the intracellular fluid, the main cations are magnesium and potassium and the phosphates and proteins are the main anions. Sodium and chloride are less in concentration. In the extracellular fluid, the predominant cation is sodium and the principle anions are chloride and bicarbonates, along with these diffusible nutrients and metabolites as glucose and urea are present in the ECF. Normally there exist a balance between the amount of water absorbed into the body and the amount eliminated. The water is absorbed through the intestines and eliminated as urine through kidneys, as sweat through skin and insensible losses through exhaled air, faeces and body secretions. The two main subdivisions of extracellular fluid – the blood plasma and interstitial fluid – are separated from each other by capillary wall which is freely permeable to water but does not allow free passage of plasma proteins resulting in higher concentration of protein content in the plasma. Besides changes in the volume of fluids in the compartments, changes in the ionic equilibrium affect acid base balance of the fluids. An acid is a molecule which gives off a hydrogen ion and a base is a molecule that takes up a hydrogen ion. During normal metabolic activity a number of acids as carbonic, phosphoric, sulphuric, lactic, hydrochloric and ketoacids are formed, but still the pH of the blood is constantly maintained at 7.4 in health and this balance is regulated by Increased volume of blood from the arterial and arteriolar dilatation is referred to as hyperemia which is effected through sympathetic neurogeneic mechanism or release of vasoactive substances and the affected organ is red in appearance, whereas impaired venous drainage is called venous congestion or passive hyperemia and the affected tissue is bluish in colour. The obstruction to the venous flow can be either systemic or local. Systemic venous congestion is caused mainly due to heart failure. Left-sided heart failure presents as chronic venous congestion of lung and right-sided heart failure as chronic venous congestion of liver and spleen (Figs 1.28.14–1.28.17). Haemorrhage is the escape of blood from a blood vessel. It may occur externally as traumatic injury or internally into the serous cavities or into the hollow viscus or into the skin and mucous membranes. Rapid loss of 33% of blood volume is more serious than gradual blood loss of 50% in 24 h. The blood loss may be large and sudden or as small repeated bleeds over a period of time. It can be due to trauma to the vessel wall, spontaneous rupture of an aneurysm, associated with bleeding diathesis, neoplastic invasion of vessel, vascular diseases or elevated blood pressure leading to vessel rupture. Definition: Thrombosis is an inappropriate activation of normal haemostasis, resulting in formation of a blood clot (thrombus) in uninjured vasculature. Both hemostasis and thrombosis depend on three general components (Fig. 1.28.18) The fully formed thrombus can undergo complete lysis or can retract, organize and recanalize or can get infected and form septic emboli. If the thrombus gets detached from the site of attachment it forms an emboli and it occludes the travelling vessel at a distant site (Fig. 1.28.20).
1.28: Basics of general pathology
Cell injury
Adaptation
Morphological changes in reversible cell injury
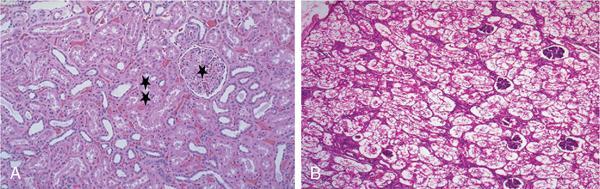
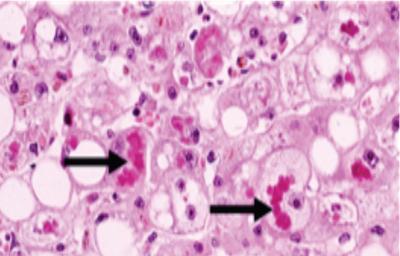
Irreversible cell injury – cell death and necrosis
Irreversible cell injury
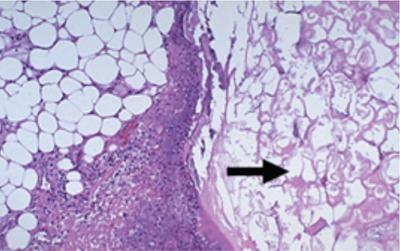
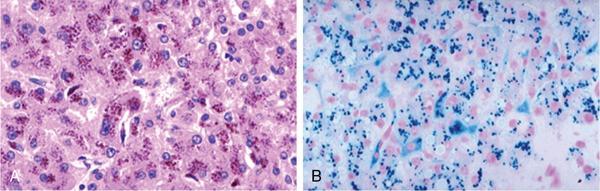
Pigment accumulation
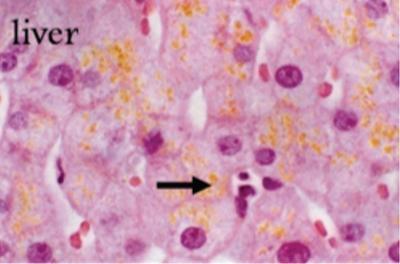
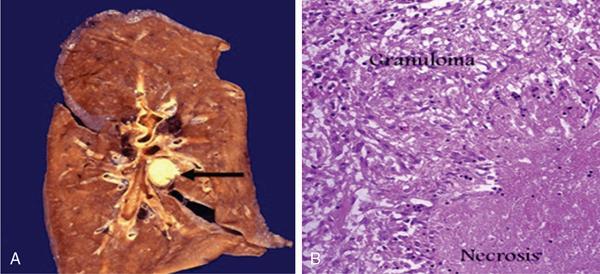
After effects of necrosis
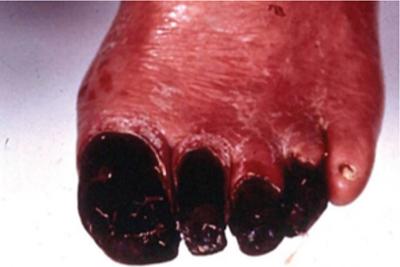
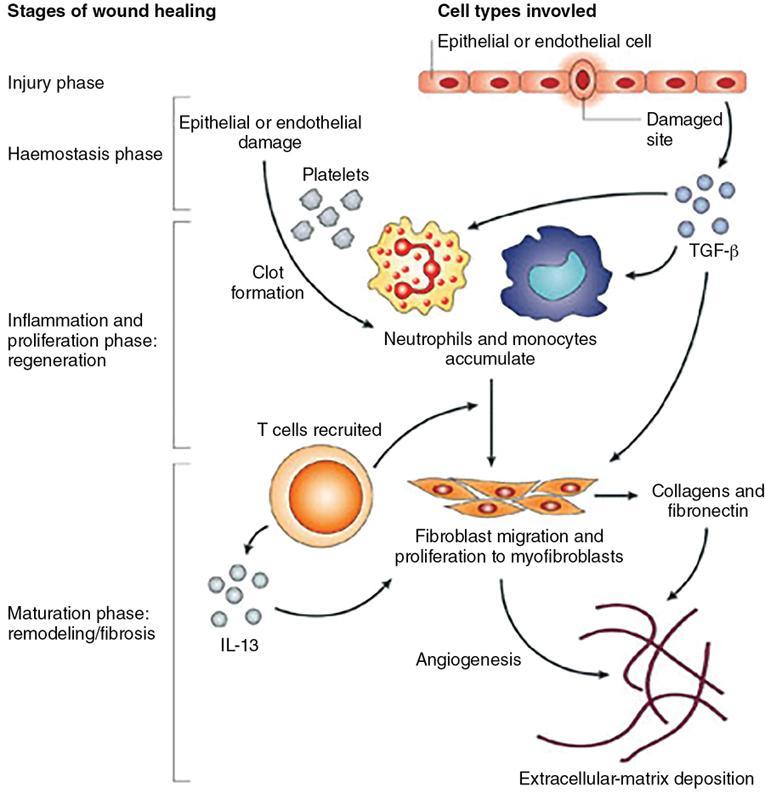
Inflammation and healing
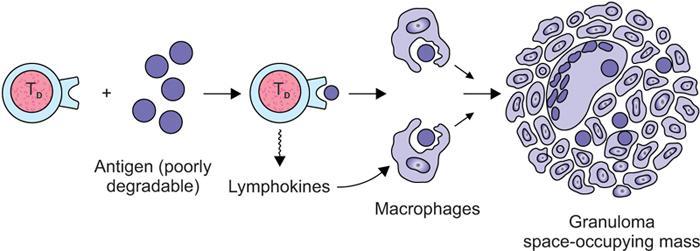
Granulomatous inflammation
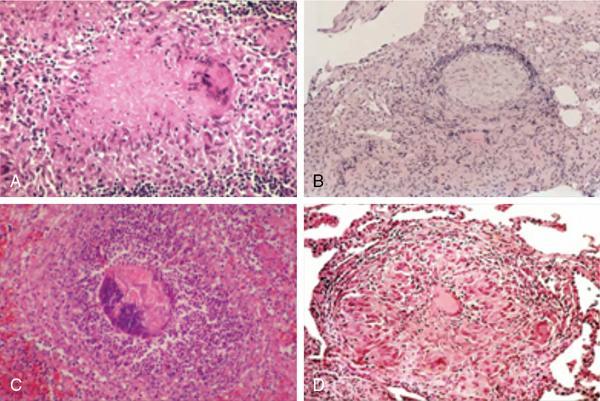
Granulomatous conditions
Mantoux test – tuberculin intradermal test
Wound healing
Healing by first intention
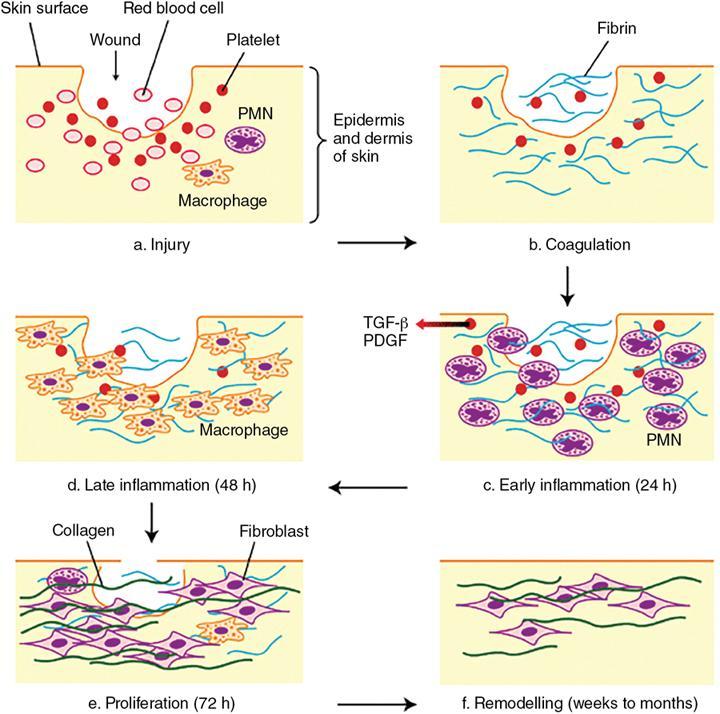
Healing by secondary intention
Wound contractures and strength by extracellular matrix
Fibrosis
Homeostasis
Normal composition of internal environment
Normal water and electrolyte balance (Gibbs donnan equilibrium)
Acid base balance
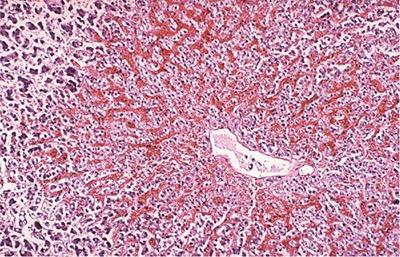
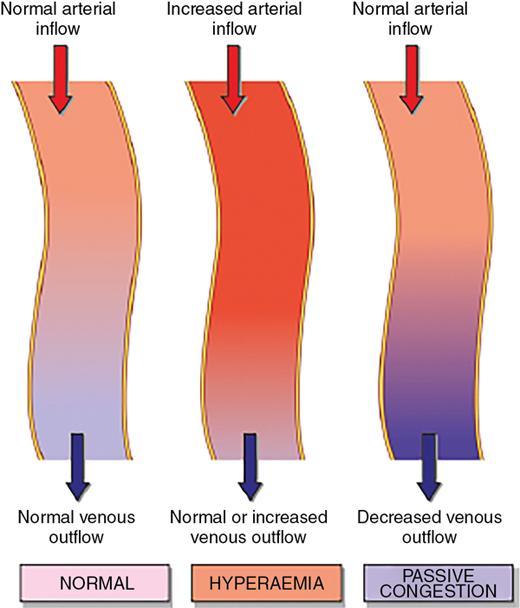
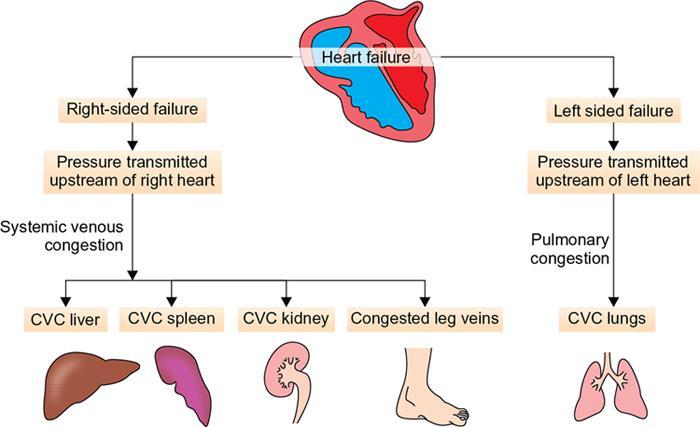
Hyperemia and congestion
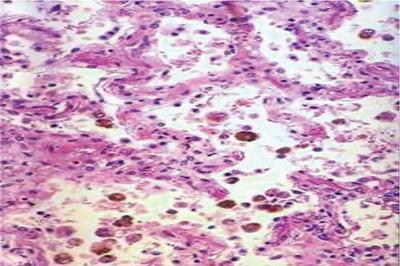
Haemorrhage
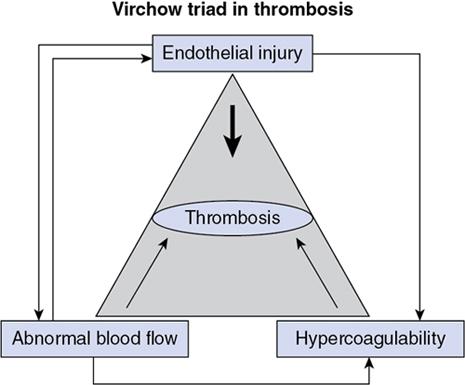
Thrombosis and infarction
High risk for thrombosis
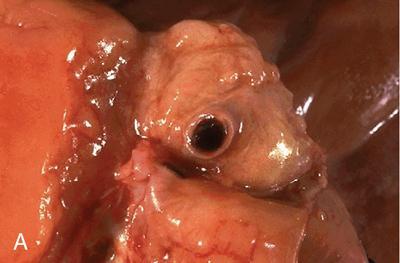
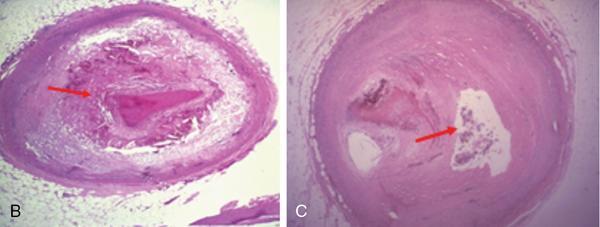
Low risk for thrombosis
Deep vein thrombosis
Five stages in the thrombus formation
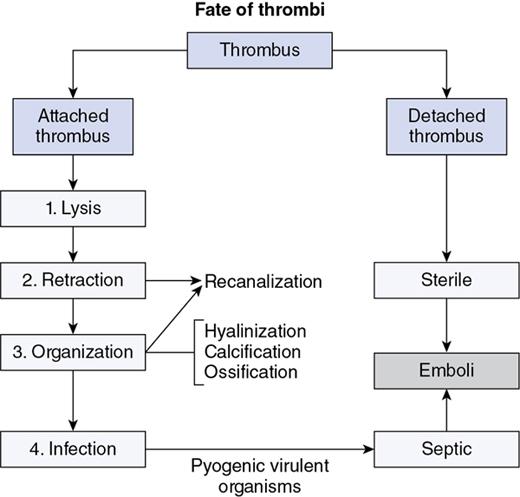
Fate of the thrombi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree