The urinary bladder is composed of the following four layers:
- 1.
Urothelium: Transitional epithelium
- 2.
Lamina propria: Vascular layer of connective tissue deep to the urothelium
- 3.
Muscularis propria: Detrusor muscle
- 4.
Adventitia: Connective tissue
The bladder is an extraperitoneal organ with a serosal (peritoneal) covering present only over the dome. The remainder of the bladder is surrounded by perivesical fat.
This chapter reviews the benign and malignant processes that can affect the urinary bladder. With a nonspecific clinical presentation, most bladder tumors have a broad differential diagnosis that includes both benign and malignant entities. Definitive diagnosis is usually established by histologic examination. Paraganglioma is the only bladder lesion with symptoms and biochemical findings that permit a specific clinical diagnosis.
The differential diagnosis is broad when imaging findings are nonspecific and includes both benign and malignant entities. Other benign lesions to consider that also may manifest as single or multiple focal masses include blood clot, endometriosis, nephrogenic adenoma, eosinophilic cystitis, malakoplakia, and infections such as tuberculosis, schistosomiasis, and fungal infections. Primary bladder carcinomas, lymphoma, and metastatic disease from adjacent or distant organs should be considered among the differential diagnoses for malignant bladder tumors. Clinical presentation, accompanying secondary imaging findings, and histologic analysis allow for more accurate differentiation from the primary benign tumors of the bladder.
Benign Bladder Lesions
Etiology
Primary tumors of the urinary bladder may arise from any of the four layers of the bladder wall, with the majority (95%) arising from the epithelial layer. By contrast, primary benign tumors of the urinary bladder arise from the submucosa, accounting for a minority (~1%) of bladder tumors. These tumors are typically mesenchymal with differentiation toward vasculature, nerve, cartilage, fat, muscle, or fibrous tissue. Mesenchymal bladder tumors include leiomyoma, hemangioma, paraganglioma, neurofibroma, inflammatory pseudosarcoma, solitary fibrous tumor, lipoma, and fibroma.
Prevalence and Epidemiology
Leiomyoma
Leiomyoma is the most common benign mesenchymal tumor of the bladder, accounting for 0.4% of all bladder tumors. Most leiomyomas occur in middle-aged women, with 76% of patients with leiomyoma being female.
Hemangioma
Bladder hemangioma, accounting for 0.3% of all bladder tumors, is typically a congenital tumor that is usually noted during childhood or adolescence but may be diagnosed at a later age as well. There may be a slight male predominance. It is most commonly observed as a single lesion, although additional hemangiomas may be observed elsewhere in the body up to 30% of the time, for example, in the skin. These tumors may occur in association with Klippel-Trenaunay-Weber and Sturge-Weber syndromes.
Paraganglioma
Paraganglioma, a pheochromocytoma occurring outside the adrenal gland, is uncommon, accounting for 0.1% of all bladder tumors. Although it may occur at any age, paraganglioma is more common among adults. There is a slight female predilection. Whereas most paragangliomas are isolated, they also may occur in conjunction with phakomatoses such as neurofibromatosis, Sturge-Weber syndrome, and tuberous sclerosis or other syndromes, including von Hippel-Lindau syndrome or multiple endocrine neoplasia types 2a and 2b. Rare observations of familial extra-adrenal pheochromocytomas also have been reported. In 5% to 15% of cases, paraganglioma of the bladder may be malignant.
Neurofibroma
Neurofibroma is a rare bladder tumor, although the urinary bladder is the most common site of involvement within the genitourinary system. These tumors occur in isolation or in association with neurofibromatosis type 1 (NF1) in 55% of cases. Neurofibromas are more common in men 20 to 40 years of age. A younger age at onset suggests association with NF1. Neurofibromas associated with NF1 are usually multiple or are of the pathognomonic plexiform type. Malignant degeneration of neurofibromas, more common in cases associated with NF1, should be suspected in tumors that rapidly increase in size or are heterogeneous in appearance.
Inflammatory Pseudotumor
Inflammatory pseudotumor is also known by its more descriptive name, pseudosarcomatous fibromyxoid tumor, which better reflects its histologic composition. This is a rare tumor that occurs at any age from childhood to late adulthood and may have a slight male predilection. First described in the lung, inflammatory pseudotumor may involve any organ. The tumor behaves aggressively and mimics malignancy on imaging. Distinction is made only by histologic examination.
Solitary Fibrous Tumor
Solitary fibrous tumor is an exceedingly rare tumor in the urinary bladder with only seven reported cases in the literature. It is most commonly seen in the thoracic cavity, where it involves the visceral pleura. Reports of extrapleural involvement in multiple other organ systems also exist. It preferentially affects men 42 to 67 years of age.
Lipoma and Fibroma
Lipoma and fibroma occur only rarely in the urinary bladder and demonstrate features in the urinary bladder similar to those seen in other more commonly involved sites in the body.
Clinical Presentation
Most patients with bladder tumors present with nonspecific urinary symptoms, such as hematuria, frequency, urgency, dribbling, or urinary obstruction, that vary depending on the size and location of the tumor. Some may be asymptomatic. Painless hematuria is a common manifestation of hemangiomas.
Patients with paragangliomas may present with episodic headaches, anxiety, sweating, tremors, and hypertension occurring with each catecholamine surge with urination—so-called micturition attacks. Hematuria is also common with paragangliomas. Twenty-four–hour urine collection analysis reveals elevated levels of metanephrines or vanillylmandelic acid. Most (83%) but not all paragangliomas are hormonally active.
Pathology
Leiomyoma
Leiomyoma is usually a solitary tumor that exhibits growth intraluminally (63%), intramurally (7%), or outside the confines of the bladder (30%). The lesion typically occurs at the bladder base near the trigone, although it also may be found on the lateral or posterior walls.
Hemangioma
Hemangioma manifests as a small, lobulated, broad-based, sessile mass in the bladder dome or posterolateral wall. Although most hemangiomas extend into the muscular layer of the bladder wall, approximately one third are located in the submucosa and a minority may also extend beyond the bladder wall. Hemangiomas also may manifest as diffuse bladder wall thickening.
The three distinct types of hemangiomas described include cavernous, capillary, and arteriovenous. The cavernous type accounts for most (78%) bladder hemangiomas, whereas capillary and arteriovenous types each represent 10% of cases.
Paraganglioma
Paragangliomas arise from the chromaffin cells of the sympathetic plexus in the bladder wall. Most are found near the trigone, although they may occur anywhere in the bladder.
Neurofibroma
Neurofibromas originate from the sheath of nerve plexuses entering near the bladder trigone. The tumor manifests as a smooth, well-defined focal intraluminal or intramural mass anywhere in the bladder. The plexiform variety manifests as a focal, nodular mass resulting in bladder wall thickening or as diffuse bladder wall thickening that involves only the inner wall, sparing the outer bladder wall. Plexiform neurofibromas also may involve surrounding structures such as the rectum, urethra, prostate, seminal vesicles, uterus, vagina, and the pelvic sidewalls.
Inflammatory Pseudotumor
The pathogenesis of this tumor is uncertain, although it is postulated to develop as a result of recurrent cystitis, inflammation, or prior surgery. However, many patients give no such prior history. This tumor usually occurs as a solitary mass ranging from 2 to 8 cm anywhere in the bladder with a tendency to spare the trigone. The solitary mass may project into the lumen, may be intramural, or may demonstrate extension into the surrounding extravesical tissues.
Solitary Fibrous Tumor
Solitary fibrous tumor may be an incidental finding or it may produce symptoms owing to its large size, which ranges from 3 to 17 cm. It may occur anywhere in the bladder.
The histopathologic features of solitary fibrous tumor may be confused with those of other tumors such as hemangiopericytoma, leiomyoma, leiomyosarcoma, and schwannoma. However, immunohistochemical analysis specifies the diagnosis because a solitary fibrous tumor strongly expresses CD34 on its surface that is not expressed as exuberantly by other tumors. Recently, this tumor has also shown positivity to BCL2, insulin-like growth factor type 2, and CD99.
Imaging
The imaging features of most benign bladder tumors are not specific. The tumor generally appears as a solid enhancing mass that is well circumscribed with smooth margins on cross-sectional imaging. However, differing histologic composition may give rise to characteristic imaging findings in certain tumors that allow greater specificity. Magnetic resonance imaging (MRI) is superior to other modalities given its ability to better characterize soft tissue as well as demonstrate the submucosal location of these tumors. Enhancing mucosa overlying the mass is seen on postgadolinium images. Because the imaging features of most primary benign bladder tumors are not specific, only those tumors with distinct imaging characteristics are discussed in the following sections.
Radiography
Intravenous urography and cystography demonstrate a filling defect or defects in the case of multiple lesions ( Figure 68-1 ). A specific diagnosis or even differentiation between benign or malignant tumors cannot be made with these techniques, and additional imaging or evaluation is required for more definitive diagnosis.

Computed Tomography
Computed tomography (CT) typically demonstrates an enhancing mass in the background of a urine-filled bladder on contrast-enhanced images and a filling defect of variable morphology in the background of a contrast-filled urinary bladder in the excretory phase of imaging.
Calcifications have not been reported in bladder leiomyomas ( Figure 68-2, A ). Although rarely observed, ring calcification around the periphery of a bladder mass is highly suggestive of a paraganglioma ( Figure 68-3, A and B ). Hemangiomas also may be associated with calcifications, which in the case of cavernous hemangiomas represent phleboliths.


Neurofibroma.
Neurofibroma appears as a homogeneous, well-defined mass of low attenuation on unenhanced images with homogeneous enhancement on postcontrast images. Scattered areas of low attenuation, reflecting myxoid degeneration, also may be seen after administration of a contrast agent. A target-like enhancement pattern, characteristic of plexiform neurofibromas, also may be observed ( Figure 68-4 ). In this pattern there are areas of high attenuation centrally, which corresponds histologically to nerve tissue, as well as areas of low attenuation peripherally, corresponding to myxoid degeneration.

Inflammatory Pseudotumor.
Inflammatory pseudotumor appears as an enhancing, solitary, polypoid mass with a central area of low-density corresponding histologically to necrosis ( Figure 68-5 ). This appearance results in peripheral enhancement surrounding a central nonenhancing area. Extravesical spread may also be noted.

Magnetic Resonance Imaging
Many tumors demonstrate low to intermediate signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. Only those tumors with distinct MRI features are discussed here.
Leiomyoma.
Because of the smooth muscle composition of leiomyomas, MRI allows for more specific characterization than other modalities. Imaging characteristics are similar to those seen with the more commonly encountered uterine leiomyomas. MRI usually demonstrates a homogeneous, well-defined mass of decreased to intermediate T1 signal and decreased T2 signal that enhances after administration of gadolinium (see Figure 68-2, B to D ). Enlarging leiomyomas may demonstrate heterogeneity and degeneration, which may be cystic in the case of bladder leiomyomas. Cystic degeneration manifests as increased T2 signal and a corresponding area of nonenhancement.
Hemangioma.
Hemangiomas give low to intermediate signal on T1-weighted images, with marked hyperintensity on T2-weighted images.
Paraganglioma.
Paragangliomas are usually hypointense on T1-weighted images. Classically, and as also observed with their adrenal counterparts, paragangliomas are hyperintense (“light bulb”) on T2-weighted images. However, only mild hyperintensity on T2-weighted images is seen in 20% of cases. There is marked enhancement after contrast agent administration.
Neurofibroma.
The highly characteristic target pattern of enhancement also may be seen with MRI. On T1-weighted images, the central portion demonstrates increased signal compared with the periphery. On T2-weighted images, the peripheral portion, corresponding histologically to myxoid degeneration, demonstrates increased signal compared with the central portion, which corresponds histologically to nerve tissue.
Inflammatory Pseudotumor.
Inflammatory pseudotumor exhibits low signal on T1-weighted images and heterogeneously high signal on T2-weighted images. Enhancement characteristics are similar to those seen on CT.
Solitary Fibrous Tumor.
Solitary fibrous tumor is a solid enhancing mass that exhibits T2 hypointensity ( Figure 68-6 ).

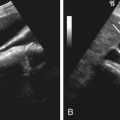
Ultrasonography
Ultrasonography shows an isoechoic to hypoechoic intramural mass with a variable degree of vascularity on color Doppler evaluation ( Figure 68-7 ). Of note, hemangiomas and paragangliomas manifest as hyperechoic masses with marked vascularity. Color Doppler evaluation of hemangiomas and paragangliomas demonstrates a high-velocity, low-resistance arterial waveform.

Nuclear Medicine
Except for paragangliomas, in which iodine-131–labeled meta-iodobenzylguanidine ( 131 I-MIBG) scan may demonstrate increased uptake in the bladder mass with a specificity of 96%, nuclear medicine plays no role in the evaluation of primary benign bladder tumors (see Figure 68-3, C ). However, sensitivity of this scan is much lower, at approximately 65%. The 131 I-MIBG scan also may be useful for evaluating the whole body for metastatic paraganglioma.
Treatment
Treatment of most benign bladder tumors is surgical, often using a cystoscopic approach. However, in the case of paraganglioma, partial cystectomy is usually performed after adrenergic blockade to prevent hypertensive crisis. Adjacent lymph node dissection is also performed if there is evidence of involvement. Some patients will undergo cystoscopic surveillance, because some tumors have a tendency to recur, such as solitary fibrous tumors, in which long-term follow-up is required because 10% to 15% of these tumors can be malignant.
- •
Benign mesenchymal tumors of the bladder are uncommon.
- •
Imaging findings, although generally nonspecific, may suggest the correct diagnosis in certain cases and further guide management.
Malignant Bladder Lesions
Etiology
Bladder cancer accounts for 2% to 6% of all new cancers in the United States. Most bladder cancers occur in men older than 65 years of age and are associated with smoking and occupational exposure to carcinogens. Bladder calculi, chronic infection, urinary stasis (e.g., within diverticula), arsenic in drinking water, and drugs such as phenacetin and cyclophosphamide have been linked with development of bladder cancer.
Urothelial cancer pathogenesis is related to prolonged urothelial contact with excreted carcinogens in the urine. Tobacco smoking is considered the biggest risk factor for developing bladder carcinoma and confers a relative risk of 4 times that of the nonsmoking population. It is estimated that 50% of bladder cancers in men are related to smoking. In addition, occupational exposure to aromatic amines in the dye industry has been proved to induce bladder cancer. Because the bladder has a proportionately higher surface area and remains in contact longer with excreted carcinogens, it is 40 times more likely to develop urothelial malignancy than the upper urinary tracts. Prolonged irritation from bladder stones and chronic infection (especially schistosomiasis) also increases risk but is more strongly associated with squamous cell bladder cancer. Drugs that have been linked with bladder cancer include phenacetin and cyclophosphamide. Although occupational exposure to aniline dyes in hair products confers increased risk, personal use of hair dye products has not been implicated. Arsenic content in drinking water and pelvic radiation therapy for cervical carcinoma also have been linked to increased risk. Familial risk is poorly understood but likely plays a limited role, as demonstrated by twin studies and family-based population analyses. Positive family history results in as much as a twofold risk for developing bladder cancer.
Prevalence and Epidemiology
In the United States, bladder cancer is the fourth most common malignancy in males and ninth most common malignancy in females. Approximately 77,000 new cases of bladder cancer are diagnosed each year, with a male predominance of 4 : 1. An estimated 16,400 deaths will be due to bladder cancer in 2016. At diagnosis, 74% of bladder cancers are localized, 19% demonstrate regional spread, and 4% present as distant metastases.
The majority of bladder cancers occur in patients older than 65 years of age, with the peak incidence between 75 and 84 years of age. Overall, 1 in 42 (2.4%) individuals will be diagnosed with cancer of the urinary bladder during their lifetime.
Bladder malignancies are broadly classified as primary or secondary, with primary lesions further divided by their histologic layer of origin. The overwhelming majority (95%) of malignant bladder lesions are primary malignancies that arise from the epithelium. These include urothelial carcinoma (90%), squamous cell carcinoma (2% to 15%), and adenocarcinoma (<2%). Nonepithelial tumors comprise only 5% of bladder cancer cases and include rhabdomyosarcoma (more common in children) and leiomyosarcoma (more common in adults). Rare bladder tumors include metastases, lymphoma, pheochromocytoma, carcinosarcoma, and malignant fibrous histiocytoma.
The Tumor, Node, Metastasis (TNM) staging system is widely used and based on the modified Jewett-Strong system ( Table 68-1 ).
| Stage | Description |
|---|---|
| PRIMARY TUMOR (T) | |
| Tx | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| Ta | Noninvasive papillary carcinoma. |
| Tis | Carcinoma in situ (i.e., flat tumor). |
| T1 | Tumor invades subepithelial connective tissue. |
| T2 | Tumor invades muscle. |
| pT2a | Tumor invades superficial muscle (inner half). |
| pT2b | Tumor invades deep muscle (outer half). |
| T3 | Tumor invades perivesical tissue. |
| pT3a | Microscopically |
| pT3b | Macroscopically (extravesical mass) |
| T4 | Tumor invades any of the following: Prostate, uterus, vagina, pelvic wall, or abdominal wall. |
| T4a | Tumor invades the prostate, uterus, or vagina. |
| T4b | Tumor invades the pelvic wall, or abdominal wall. |
| REGIONAL LYMPH NODES (N) | |
| Nx | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastasis. |
| N1 | Metastasis in a single lymph node ≤2 cm in largest dimension. |
| N2 | Metastasis in a single lymph node >2 cm but ≤5 cm in largest dimension; or multiple lymph nodes ≤5 cm in largest dimension. |
| N3 | Metastasis in a lymph node >5 cm in largest dimension. |
| DISTANT METASTASIS (M) | |
| Mx | Distant metastasis cannot be assessed. |
| M0 | No distant metastasis. |
| M1 | Distant metastasis. |
| AJCC STAGE GROUPINGS | |
| Stage 0a | Ta, N0, M0 |
| Stage 0is | Tis, N0, M0 |
| Stage I | T1, N0, M0 |
| Stage II | T2a, N0, M0 T2b, N0, M0 |
| Stage III | T3a, N0, M0 T3b, N0, M0 T4a, N0, M0 |
| Stage IV | T4b, N0, M0 Any T, N1, M0 Any T, N2, M0 Any T, N3, M0 Any T, any N, M1 |
Clinical Presentation
Hematuria, either gross or microscopic, is the presenting symptom in more than 80% of cases. Other symptoms include dysuria, increased frequency, and/or pelvic pain and pressure. Obstructive uropathy related to bladder cancer usually occurs in the setting of muscularis propria invasion. Typical symptoms of bladder outlet obstruction are often absent, owing to the slowly progressive nature of the tumor.
Pathology
Urothelial carcinoma, a term now preferred over the previously used transitional cell carcinoma, accounts for over 90% of epithelial bladder malignancies. Substantial overlap exists in the imaging characteristics of different subtypes of malignant bladder lesions. Lesions originating from the urothelium typically have a papillary or nodular appearance ( Figure 68-8 ). They also can manifest as flat or plaque-like lesions such as squamous cell carcinoma, which tends to be more sessile rather than the typical papillary appearance of urothelial carcinoma. Alternatively, lesions that originate from the muscularis propria, such as rhabdomyosarcoma, can have a lobulated appearance sometimes referred to as “sarcoma botryoides,” akin to a cluster of grapes. Urachal adenocarcinoma typically manifests as a large (mean diameter, 6 cm), often partially calcified, infraumbilical soft tissue mass with disproportionate extravesical component ( Figure 68-9 ) compared with other bladder neoplasms.
Bladder cancer most commonly arises in the lateral bladder walls and less commonly along the trigone. The bladder dome is the least common site, except in cases of urachal carcinoma (see Figure 68-9 ). Bladder diverticula, when present, have a higher incidence of cancer than the remainder of the bladder, presumably owing to urinary stasis ( Figure 68-10 ).